Drugstores to Start Selling Over-The-Counter Narcan

For the first time, people across the US will be able to purchase an overdose-reversal drug that’s as easy to administer as Flonase, without a prescription.
Next week, nationwide chains like Walgreens, CVS, Walmart, and Rite Aid will begin selling two-dose boxes of Narcan, a naloxone nasal spray that saves people from opioid overdose, in stores and online.
Making naloxone widely accessible has long been a goal for public health experts because Fentanyl-laced drugs can kill people before paramedics arrive, but some now worry that over-the-counter Narcan’s $45 retail price could be too high for those who need it most.
That’s where insurance (kind of) comes in:
- Medicaid and Medicare already cover prescription naloxone, and so far, Missouri, California, Massachusetts, Washington, Rhode Island, and Oregon Medicaid programs said they’ll cover OTC Narcan, too.
- While private health plans often restrict OTC drug coverage, Blue Cross Blue Shield of Massachusetts said it’ll fully cover nonprescription Narcan.
But this won’t help the one-fifth of people with opioid use disorder who are uninsured. Some government and harm reduction programs give out Narcan for free—and those groups can now order two-dose boxes in bulk at a discounted $41 per box, according to manufacturer Emergent BioSolutions.
Other options include…a prescription-only naloxone generic by Teva Pharmaceuticals, which is widely available for less than $10 out of pocket. And another OTC version, RiVive, will head to stores early next year and is expected to cost less than Narcan.
Save the Date: CMS Staff to Talk Making Care Primary Model With Interested Candidates

The Centers for Medicare and Medicaid Services (CMS)recently released the request for applications for its new Making Care Primary Model, which is set to start operating July 2024 in eight states, including North Carolina. Applications for the model open on September 4 and close November 30. Additional information can be found in our recent advocacy update article.
The full article from NCMS can be found by clicking here.
In preparation, the AMA is collaborating with ACP and AAFP to host an informative webinar through which AMA members interested in applying will be able to engage directly with CMS staff.
CMS Model Lead staff will open the webinar with a brief overview of the model followed by a robust Q&A where they will answer your questions live.
Additional details including a formal registration link will follow, but for now please save the date for Wednesday, September 27th at 7-8 pm ET.
Please reach out to [email protected] with any immediate questions, otherwise stay tuned for more information.
Biden-Harris Administration Announces First Ten Drugs Selected for Medicare Price Negotiation
(Reuters) -- The Biden administration on Tuesday released its list of 10 prescription medicines that will be subject to the first-ever price negotiations by the U.S. Medicare health program that covers 66 million people, with big-selling blood thinner Eliquis from Bristol Myers Squibb (BMY.N) and Pfizer (PFE.N) among them.
President Joe Biden’s signature Inflation Reduction Act (IRA), signed into law last year, allows Medicare to negotiate prices for some of its most costly drugs.
"Today is the start of a new deal for patients,” Biden said at a White House event, adding that Americans often pay two to three times more than other countries for the same drugs.
Once implemented, the prices on negotiated drugs will decrease for up to 9 million seniors who currently pay as much as $6,497 in out-of-pocket costs per year for these prescriptions, Biden said.
Merck & Co's (MRK.N) diabetes drug Januvia, Eliquis rival Xarelto from Johnson & Johnson (JNJ.N), and AbbVie's (ABBV.N) leukemia treatment Imbruvica were also selected.
Other medicines picked for negotiations include Amgen's (AMGN.O) rheumatoid arthritis treatment Enbrel, Boehringer Ingelheim and Eli Lilly's (LLY.N) diabetes drug Jardiance, J&J's arthritis and Crohn's disease medicine Stelara and insulin from Novo Nordisk (NOVOb.CO).
Medicare, which mostly serves Americans aged 65 and over, pays twice as much for drugs than the U.S. Department of Veterans Affairs, which already negotiates drug prices, he said.
Shares of drugmakers were mixed on Monday afternoon. The NYSE Arca Pharmaceutical index (.DRG) was up around 0.4%.
Tuesday's announcement kicks off the negotiation process for the 10 drugs whose new prices will go into effect in 2026. The program aims to save $25 billion per year on drug prices by 2031.
U.S. laws had prohibited Medicare from negotiating pharmaceutical prices as part of its prescription drug program that began about 20 years ago.
The U.S. Centers for Medicare & Medicaid Services (CMS) spent $50.5 billion between June 1, 2022 and May 31, 2023 on the 10 drugs, which is the time period used to determine which medicines were eligible for negotiation. That was about 20% of the total cost of drugs in the Medicare prescription drug program known as Part D.

Wells Fargo analyst Mohit Bansal said the savings from negotiations on Jardiance, Januvia, AstraZeneca's (AZN.L) Farxiga and Novo's Insulin Aspart, which cost the agency about $16.5 billion total, could potentially free up Medicare's budget and make it easier to cover diabetes or obesity drugs.
CLINICALLY APPROPRIATE ACCESS
Novartis , whose heart failure drug Entresto was among the 10 selected, Eli Lilly and Merck said they believed the price-setting would stifle innovation in the sector and impact quality of care. AstraZeneca and Novo Nordisk said they were evaluating next steps.
Bristol Myers CEO Giovanni Caforio in an interview said the inclusion of Eliquis would not impact its long-term strategy, particularly as the drug loses patent exclusivity in 2028, two years after the negotiated prices would take effect.
Caforio said Medicare enrollees on the drug could see their access restricted because of unintended consequences of the law.
"There is no requirement in the law that insurance companies that administer Medicare benefits will actually continue to make these medicines available to patients without hurdles or burdensome cost sharing," he said.
CMS Director Dr. Meena Seshamani said Medicare plans to use a review process to make sure insurance companies keep clinically appropriate access to negotiated drugs.
Competition for J&J's Stelara is expected to hit the U.S. market in 2025, before negotiated prices go into effect, following deals with Amgen, Alvotech (ALVO.O) and Teva (TEVA.TA) that delayed launches of their near copies, known as biosimilars.
Analysts had said the delays put J&J back on track for $57 billion in 2025 pharmaceutical revenue.
Bristol Myers, J&J, Merck, AstraZeneca, and Germany-based Boehringer have also sued the U.S. Department of Health and Human Services, which oversees the Medicare agency, in an effort to derail the price-setting process.
BMO Capital Markets analyst Evan Seigerman said that while the list includes many big revenue generators, many of them will face competition shortly after or even before 2026, which was expected to lessen their profitability.
Two analysts said they expect the negotiated prices to move beyond Medicare and affect commercial markets for these drugs by 2026, when they come into effect.
The 10 initial drugs were chosen based on certain criteria set out by Medicare. They must be sold in pharmacies, not have substantial generic competition, and have been on the market for at least nine years - 13 for more complex biotech drugs.
Reporting by Patrick Wingrove, Mike Erman, Manas Mishra in Bengaluru and Nandita Bose in Washington; Editing by Caroline Humer, Bill Berkrot and Chizu Nomiyama
Additional Reading:
Physicians Foundation 2024 Fellowship Program Now Accepting Applications


The Physicians Foundation recently launched a call for applicants for the Fellowship Program! New or early-career physicians are encouraged to apply by September 19.
With a focus on drivers of health (DOH), the Fellowship Program enhances physicians’ leadership skills so they can strengthen the physician-patient relationship, support medical practices’ sustainability and navigate the changing health care system. The fellow will develop and implement a project that supports physicians in their understanding of DOH and integration into medical practices.
Requirements include:
- Open to physicians who are new or early-career in their practice.
- Applicants must demonstrate experience with and/or interest in DOH, physician leadership, health policy, health equity and clinical/care delivery innovation.
- Submit a program application with a current resume/CV and statement of intent that briefly describes how participation will be a transformative experience for you to learn how to make positive, constructive contributions to the medical profession in the future.
- Receive two (2) letters of recommendation, one of which is recommended to be from the fellow's state or county medical societ
TO APPLY FOR THE FELLOWSHIP CLICK HERE
About The Physicians Foundation
The Physicians Foundation is a nonprofit seeking to advance the work of practicing physicians and help them facilitate the delivery of high-quality health care to patients. As the U.S. health care system continues to evolve, The Physicians Foundation is steadfast in strengthening the physician-patient relationship, supporting medical practices’ sustainability and helping physicians navigate the changing health care system. The Physicians Foundation pursues its mission through research, education and innovative grant making that improves physician wellbeing, strengthens physician leadership, addresses drivers of health and lifts physician perspectives. For more information, visit www.physiciansfoundation.org.
NCDHHS: NC Medicaid Expansion Will Not Launch October 1 as Scheduled
NC Medicaid Expansion Will Not Launch on October 1 as Scheduled

(NCDHHS) -- In March, overwhelming bipartisan majorities in the General Assembly passed Medicaid expansion, finally putting us on a path to close the coverage gap and enable access to care for more than 600,000 North Carolinians. Since that time, NCDHHS has been completing the extensive policy and technical work necessary to launch expansion and working with our partners to be ready on day one. However, the General Assembly tied authority to implement Medicaid expansion to the state budget’s enactment. With budget discussions delayed, last month NCDHHS announced that if we received final authority to move forward with expansion by September 1 – either through the budget or separate legislation – we would be able to go-live with expansion on October 1. It is now clear that the General Assembly will neither pass a budget nor authorize NCDHHS to launch Medicaid expansion by this September 1 deadline. Therefore, Medicaid expansion will not launch on October 1 as we had hoped.
With the continued uncertainty around when final authority will be granted to NCDHHS, we are unable to set a new launch date at this point. We are eager to move forward with Medicaid expansion implementation as quickly as possible and while we had previously announced that the next likeliest date for expansion go-live would be December 1, we will wait for more clarity around final authority before setting a definitive date. We recognize county partners and community stakeholders have invested greatly in their readiness and the department encourages them to stay the course. We remain committed to working together to support our partners to ensure we can leverage Medicaid expansion to increase access to care across the state.
This delay is disappointing and will tragically result in more than 600,000 North Carolinians not being able to access care when they may need it most, especially with Continuous Coverage Unwinding causing nearly 9,000 people each month to lose full health care coverage that would qualify under expansion. Nearly half of the people eligible for expansion – 300,000 people – would have been automatically enrolled in full coverage under Medicaid expansion on October 1 but now have to wait. Each month of delay costs the state hundreds of millions of dollars flowing into communities across the state to support care and treatment for people and keep providers’ doors open. Unfortunately, this delay will further strain providers who were gearing up to serve those newly eligible.
Despite this delay, NCDHHS is committed to working with our partners to prepare to enroll eligible North Carolinians on day one.
NCDHHS appreciates the leadership of the North Carolina General Assembly and Governor Roy Cooper in securing the passage of Medicaid expansion over five months ago. We hope that we receive the authority to move forward with this historic opportunity to improve the health of North Carolinians as soon as possible.
Biden Gears Up for Re-Election By Focusing on Healthcare Costs

(New York Times - David Leonhardt, Ian Prasad Philbrick) -- As his re-election gets underway, President Joe Biden is signaling that healthcare, and particularly, the cost of it, will be central to his campaign.
"We’re taking on powerful interests to bring your health care costs down,” he has said. “I’m just tired of seeing Americans ripped off,” he said last month. Tomorrow, he will hold an event at the White House at which he is expected to announce the first 10 drugs that will be part of a new program in which Medicare officials can negotiate with pharmaceutical companies to reduce drug prices.
Biden is emphasizing the cost of health care partly because it has been one of his administration’s biggest priorities, even if other policies — such as those on the climate and infrastructure — receive more attention. His administration has reduced the cost of hearing aids, reduced the cost of health insurance for people who buy it though an Obamacare exchange and reduced an array of expenses for Medicare recipients.
"Millions of people benefit from the health care provisions,” Larry Levitt, an executive vice president at KFF, a health care research group, told us. Some people, he added, will save “a lot of money.”
Biden and his aides understand that these policies are popular with swing voters, who, as this newsletter has described before, tend to lean left on economic issues while being more conservative on many social issues. That’s particularly true of swing voters who don’t have a four-year college degree. The president has described his health care policies as part of “Bidenomics in action.”
"If you look at the polling data, it’s overwhelmingly popular, what we’ve proposed,” Biden said earlier this year. “Matter of fact, it’s a hell of a lot more popular than I am.”
Today’s newsletter delves into the specifics — and some of the criticisms — of Biden’s health care agenda.
The Medicare Provisions
The Inflation Reduction Act — a law that Biden signed last year, centered on clean-energy funding — also includes measures to lower drug costs for Medicare recipients. Virtually every American age 65 and older is on Medicare, and many will save hundreds of dollars a year in out-of-pocket expenses. Those who spend the most on drugs will likely save a few thousand dollars a year, according to KFF.
Where do those savings comes from?
- As of this past January, the law caps a Medicare recipient’s out-of-pocket spending on insulin at $35 per month. The cap will save about 1.5 million Americans almost $500 a year on average.
- A provision allows Medicare recipients to receive some vaccines, like those for shingles and tetanus, at no charge.
- The law caps a recipient’s total spending on prescription drugs at $2,000 a year, although the provision will not take effect until 2025, after the upcoming presidential campaign.
- The law penalizes pharmaceutical companies that increase drug prices faster than the overall rate of inflation; in recent years, half of the drugs Medicare covered would have qualified. The law also includes the policy that allows Medicare officials to negotiate with pharmaceutical companies to reduce prices.
Those last couple of provisions will probably have a bigger effect on the government’s health care spending than on household spending. But less government spending still benefits Americans in the long run by reducing the need for taxes.
Beyond Medicare
People under 65 don’t benefit as much from Biden’s agenda, but many do benefit to some degree:
- The Inflation Reduction Act increased tax subsidies for Americans who get health insurance through the Obamacare exchanges. More than 13 million people will each save about $800 a year on average. The policy is temporary, expiring in 2026.
- An executive order that Biden signed in 2021 led the F.D.A. to allow drugstores and other retailers to sell hearing aids over the counter. The change reduced the cost of hearing aids by more than half. Before the change, the average cost of hearing aids was about $5,000.
- Biden is trying to close some loopholes that some hospitals and insurers have used to continue sending large, unexpected bills, despite a bipartisan 2020 law, signed by Donald Trump, to prevent the practice.
Potential Problems
The underlying reason for Biden’s push is that Americans pay more for medical care than the citizens of any other county. There are several causes, but many experts believe that the main factor is simply that the U.S. government doesn’t prevent drug companies, hospitals and insurers from charging high prices. An influential 2003 article in an academic journal made this argument bluntly in its headline: “It’s the prices, stupid.” These high prices translate into higher profits for health care companies.
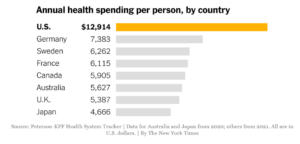
Still, there are some potential advantages to the U.S. system. High prices create an incentive for companies to develop new drugs, some of which save lives. Four of the world’s five largest drug companies are based in the U.S. The pharmaceutical industry warns that lower Medicare prices may hamper innovation, although many independent analysts — including those at the nonpartisan Congressional Budget Office — predict any such effect will be minor.
Another possible unintended consequence is that drug companies will respond to lower Medicare prices by increasing prices for people on private insurance. If that happened, it would effectively shift money from younger Americans to older Americans, and would probably worsen economic inequality.
Most experts are also relatively sanguine about these potential downsides. But, as Levitt told us, “The truth is, we don’t know.”
For More
- Biden has suggested that if he wins re-election, he will try to take more steps to reduce medical costs. Among them: making Obamacare subsidies permanent and capping insulin costs for privately insured Americans. “There’s more to do,” he has said.
- Medicare will negotiate with drug companies over the first 10 drugs next year, HuffPost’s Jonathan Cohn explains. The negotiated prices will take effect in 2026.
- Some older Americans are being charged hundreds of dollars for R.S.V. vaccines.
On The Lighter Side – August 25, 2023 - Tennis, Packapalooza, and Watermelon!
Here are some things NCMS employees, members, and YOU are talking about this week!
Djokovic, Sabalenka, Alcaraz, or Siatek? (If you know these names, you know it is US Open time!)

The main draw of the 2023 US Open doesn’t start until Monday, Aug. 28—but there’s plenty of fun to be had at the USTA Billie Jean King National Tennis Center during US Open Fan Week, which kicks off on Tuesday, Aug. 22 and runs through Sunday, Aug. 27.
During Fan Week, the grounds are open to the public, and visitors can experience a variety of fun, tennis-centric activities for newbies and die-hard fans alike. From exhibition matches featuring top players and the US Open Qualifying Tournament to a concert headlined by a Grammy-nominated singer, there’s something for everyone.
With so much to do, here are five things you should know about Fan Week before you jump on the 7 train to Flushing Meadows. Look for more on the tournament next week in OTLS!
Concerts Are Still Going Strong!

Today
- Parker McCollum, Skyla Credit Union Amphitheatre, Charlotte
- Bret Michaels & Warrant, Elmwood Park, Roanoke, VA
- Bronco, Martin Marietta Center, Raleigh
- Bill Bellamy, Parkside Main, Cary
Tomorrow
- 3 Doors Down, Red Hat Amphitheater, Raleigh
- Nickelback, Jiffy Lube Live, Bristow, VA
- The Isaacs, Liberty Showcase, Liberty
- Calvin Richardson, Rocky Mount Event Center, Rocky Mount
- The Castaways, Blackmon Amphitheater, Mt. Airy
Sunday
- Shakti, Koka Booth Amphitheater, Cary
- Pepe Aguilar, PNC Arena, Raleigh
- Clutch, The Fillmore, Charlotte
- Adam Hood, Bird's Nest, Dunn
NCSU is Back! Time for Packapalooza 2023!

An all-day block party and street festival that caps off Wolfpack Welcome Week, commencing the academic year with a bang. NC State students, faculty, staff, alums, families and members of the local community pack Hillsborough Street for a day filled with music, entertainment, family-friendly activities, food and everything that embodies the Wolfpack spirit.
All you need to know to attend is here.
Beat the Heat at the Winterville Watermelon Festival

The legendary Winterville Watermelon Festival is a multi-day event featuring activities the whole family can enjoy. You can hear local and nationally known music acts throughout the event. One of the biggest events of the festival is the Watermelon Parade through town. The watermelon-eating contest is always a favorite. Festival-goers young and old can enjoy carnival rides and midway games all weekend. In addition, visitors can check out the flea market and commercial vendors set up through the festival grounds. Food vendors serve both watermelon treats and classic fair food such as funnel cakes, turkey legs, and corn dogs. The Winterville Watermelon Festival has so much to offer. You don’t want to miss it!
All you need to know to enjoy is here!
If you have an event you would like added to On The Lighter Side, email Randy Aldridge at [email protected].
You are Invited! "Someone Else's Shoes" Tells the Story of St. Agnes Hospital

Someone Else's Shoes is a documentary highlighting trailblazers in medicine and dentistry in North Carolina who broke down barriers and paved the way for future generations, helping to increase everyone’s access to care.
The makers of the documentary say that, today’s doctors of color stand on the shoulders of giants like Bishop Henry Beard Delany ,who saved one of the largest hospitals for Blacks on the East Coast in the early 1900s,
Dr. Paul McGill, the first Black orthodontist in Charlotte, Dr. Kenneth Chambers, one of the first board-certified Obstetrician-Gynecologists in Charlotte, and Dr. Brenda Armstrong who is one of the first black students at Duke Medical School. Through in-depth interviews with experts, archival footage and images, the film tells the story of these pioneers and the challenges they faced in their pursuit of equal access to quality health care.
The film focuses on the establishment of St. Agnes Hospital in Raleigh. St. Agnes as one of the first Black-owned and operated hospitals in North Carolina. The hospital provided vital medical services to the Black community and helped to address the many challenges they faced, including discrimination and inadequate access to health care.
The Premiere Screening is Saturday, September 23, at 7:30pm (doors open at 7). You can enjoy the film at Saint Augustine's University Seby B. Jones Auditorium in Raleigh.
 The film was screened at the North Carolina Medical Society, where producer Dr. Cheryl Walker-McGill met with NCMS CEO Chip Baggett.
The film was screened at the North Carolina Medical Society, where producer Dr. Cheryl Walker-McGill met with NCMS CEO Chip Baggett.
For tickets click here.
Long Covid Lingers Longer Than You May Realize
A new study sheds some light on the condition’s lasting complications.

(Tess Bonn) -- You may have recovered from Covid-19 in a few days or weeks, but many people continue to feel the impacts of the virus for years. New findings released on Monday suggest that people who endured even mild cases of the virus are at higher risk two years later for symptoms of long Covid, and this affected their life expectancy overall.
In a study published in the journal Nature Medicine, researchers tallied roughly 80 health complications typical of the post-infection condition, like heart problems and blood clots, which translate into disability-adjusted life years (DALYs) — or time lost due to ill health. That means long Covid has a higher disease burden than even heart disease or cancer, which cause about 52 and 50 DALYs for every 1,000 Americans, according to the Institute for Health Metrics and Evaluation’s Global Burden of Disease.
This no doubt puts an immense burden on an already strained healthcare system. According to the Centers for Disease Control, nearly one in five Americans still have long Covid. The good news is it looks like these rates are declining: The number of people who say they either currently have or had symptoms from it dropped from 35 percent in June 2022 to 28 percent in January 2023, according to an analysis by the nonprofit KFF.
Wondering if you have the mysterious illness? Here’s more on what we know about the condition and the findings of the new research.
What is long Covid?
Scientists are still trying to make sense of long Covid. So far, more than 200 symptoms have been with associated with it. What we do know is that term is used to refer to new, returning, or even ongoing complications that people develop after being infected by the coronavirus.
While most people fully recover from Covid-19 within a few days or weeks, others may continue to experience symptoms of the virus. (Per the CDC, long Covid is usually defined as at least four weeks after getting sick.) Certain strains could also even put you at risk. For instance, immunologist Akiko Iwasaki told The Washington Post that the Omicron variant, which has spawned recent subvariants like XBB.1.16, is known to cause long Covid.
But not everyone who experiences long Covid knows they had the disease in the first place because they may not have had symptoms.
“Long Covid is not one illness,” the CDC states. “Your healthcare provider considers a diagnosis of Long Covid based on your health history, including if you had a diagnosis of Covid-19 either by a positive test or by symptoms or exposure, as well as doing a health examination.”
What did the long Covid study find?
One of the things the study found was that there were long-term risks associated with catching Covid-19 — even if it wasn’t a serious case. Even people who fought off mild infections were still more likely to die about six months after they first got sick than those who weren’t infected. Though their risk of having many Covid symptoms went down, it remained high for about one-third of the 77 conditions measured in the study. Some of those issues included everything from cardiovascular and gastrointestinal trouble to fatigue and trouble sleeping.
But the Covid survivors who had been hospitalized experienced much worse. They remained at increased risk for at least two years after their infection from death, subsequent hospitalization, and two-thirds of the medical conditions included in the analysis, such as diabetes to kidney disorders. They were also more likely to struggle with substance abuse and to report contemplating suicide.
“It appears that the effects of long Covid for many will not only impact such patients and their quality of life, but potentially will contribute to a decline in life expectancy, and also may impact labor participation, economic productivity, and societal well-being,” senior author Ziyad Al-Aly stated in the study.
How did researchers come up with these findings?
Researchers looked at medical records of nearly 139,000 veterans diagnosed with Covid-19 early in the pandemic in 2020, and compared them to another group of nearly 6 million veterans not known to be infected with Covid during that time.
But the Nature Medicine paper acknowledged some of its limitations. Those involved in the study were mostly in their 60s, and almost 90 percent of them were male, so they weren’t representative of who is most likely to develop long Covid. (According to a study published in the National Library of Medicine, women have a 58 percent higher risk of developing the illness compared to the rest of the population).
The study was also conducted before Covid vaccines had been developed and before the population had been able to build up immunity from infections. “The whole landscape has evolved,” coronavirus researcher Eric Topol told The Washington Post.
Back to School Time: Recommended Vaccines for Children and Teens
Time to Get Up to Date on Required and Recommended Vaccines.
RSV Vaccine Recommended for Youngest Children.

RALEIGH -- As children across North Carolina head back to school this month, the North Carolina Department of Health and Human Services reminds families that vaccinations are an important part of back-to-school success and overall health and well-being.
"Vaccines are an essential piece of both child and family health and well-being," said Dr. Zack Moore, State Epidemiologist. "We encourage parents and guardians to work with their children’s doctor to make sure their children are current on their childhood vaccines to prevent illness and reduce days missed at school."
Vaccine-preventable diseases, such as meningitis, measles, pertussis (whooping cough) and others are still seen across North Carolina. Keeping children up to date on vaccinations is the best way to keep them healthy and reduce severe illness and unnecessary absences from school. Children who are uninsured can still be vaccinated at low or no cost through the Vaccines for Children program, which offers free vaccines to eligible children through 19 years of age.
In addition, a new tool will be available this year to help protect the youngest North Carolinians from respiratory syncytial virus (RSV), the leading cause of hospitalization in the first year of life. CDC is recommending a new immunization starting this fall to help protect all infants under 8 months and some older babies at increased risk of severe illness caused by RSV.
Teens also face unique risks related to communicable diseases.
"Vaccines are one of the most effective means available for preventing the spread of disease," said Dr. Susan Kansagra, Director of the NCDHHS Division of Public Health. "They help protect the health of children and families as well as the health of the entire community. You can use any health care visit, including for sports physicals, school health assessments, check-ups, and sick visits to receive vaccines."
NCDHHS encourages all parents to talk with their child’s health care provider about recommended vaccinations. During that same visit, parents can talk with their physician about the importance of the COVID-19 vaccine and the flu vaccine for their children ages 6 months and older. Visit MySpot.nc.gov for more information about COVID-19 vaccines. Parents of infants should also talk to their pediatricians about new options for preventing severe RSV.
Governor Roy Cooper proclaimed August as Immunization Awareness Month in North Carolina. Alongside the proclamation, NCDHHS is partnering with health care providers and stakeholders in a statewide awareness campaign to help ensure school-age children are protected from vaccine-preventable diseases. Alongside the proclamation, NCDHHS is partnering with health care providers and stakeholders in a statewide awareness campaign to help ensure school-age children are protected from vaccine-preventable diseases.
More information and resources for parents and guardians is available on the CDC website:
- List of all vaccines required for school attendance, from kindergarten through 12th grade.
- CDC’s immunization catch up schedule.
- Parents unsure which vaccines their children need at any age, can find out what is needed by taking a short quiz on the CDC website.
- Information about the new RSV vaccine to prevent severe disease in infants.
- Additional information on vaccine-preventable diseases and immunizations for North Carolina families is available at immunize.nc.gov/family.
Walter B. Jones Addiction Treatment Center in Greenville Ranked One of Best in America by Newsweek

The most recent rankings were released on Newsweek.com on Aug. 16, 2023, and are based on Nationwide Online Survey, Quality Score and Accreditation Data. This is the second year in a row that WBJ has been recognized as one of the best treatment centers in North Carolina.
"Walter B. Jones is proud to be recognized again as providing top care for North Carolinians in need of substance use treatment," said Karen Burkes, NCDHHS Director of the Division of State Operated Healthcare Facilities. "Maintaining this top designation reflects the quality care provided by WBJ’s team of dedicated psychiatrists, nurses and therapists, and the support the facility receives from the community."
WBJ provides inpatient and outpatient services including medical detox, psychiatric stabilization, substance use disorder and mental health treatment, and uses best practices such as Motivational Interviewing, trauma informed care and Seeking Safety. Perinatal services are also available for women at all stages of pregnancy, from all 100 counties, who are in need of specialized inpatient treatment.
Referrals primarily come from outpatient providers, regional acute care hospitals, emergency departments, community hospitals and Local Management Entities/Managed Care Organizations. WBJ also partners with ECU Health as an integral, academic medical training institution to make sure providers are well versed in substance use treatment. Additional information about WBJ can be found at www.ncdhhs.gov/divisions/dsohf/walter-b-jones.
About 1.2 million people in North Carolina have a substance use disorder, and the COVID-19 pandemic brought added and unprecedented stressors on people, families and communities which exacerbated existing substance use issues. Having quality treatment centers like WBJ and increasing access to prevention, treatment and recovery services are crucial. And key to increasing access to behavioral health services is expanding Medicaid, which will provide millions of dollars for behavioral health services in North Carolina. Once implemented, thousands of people will have access to affordable mental health and substance use disorder treatment when and where they need. North Carolina has never had adequate mental health services because they are unaffordable for most people. Until North Carolina closes the coverage gap and implements Medicaid expansion, needed care will remain out of reach.
Walter B. Jones Center is one of two state operated Alcohol and Drug Abuse Treatment Centers specifically designed to provide medically monitored detoxification/crisis stabilization, and short-term treatment preparing adults with substance use and co-occurring disorders for ongoing community-based recovery services.
Walter B. Jones and Julian F. Keith ADATCs are operated by the NC Department of Health and Human Services-Division of State Operated Healthcare Facilities.
FDA Approves RSV Vaccine for Pregnant Women. Next Stop: CDC.
Next step to protect babies from the scary respiratory infection.

WASHINGTON (AP -- LAURAN NEERGAARD) — U.S. regulators on Monday approved the first RSV vaccine for pregnant women so their babies will be born with protection against the scary respiratory infection.
RSV is notorious for filling hospitals with wheezing babies every fall and winter. The Food and Drug Administration cleared Pfizer’s maternal vaccination to guard against a severe case of RSV when babies are most vulnerable — from birth through 6 months of age.
The next step: The Centers for Disease Control and Prevention must issue recommendations for using the vaccine, named Abrysvo, during pregnancy. (Vaccinations for older adults, also at high risk, are getting underway this fall using the same Pfizer shot plus another from competitor GSK.)
“Maternal vaccination is an incredible way to protect the infants,” said Dr. Elizabeth Schlaudecker of Cincinnati Children’s Hospital, a researcher in Pfizer’s international study of the vaccine. If shots begin soon, “I do think we could see an impact for this RSV season.”
RSV is a coldlike nuisance for most healthy people but it can be life-threatening for the very young. It inflames babies’ tiny airways so it’s hard to breathe or causes pneumonia. In the U.S. alone, between 58,000 and 80,000 children younger than 5 are hospitalized each year, and several hundred die, from the respiratory syncytial virus.
Last year’s RSV season was extremely harsh in the U.S., and it began sickening tots in the summer, far earlier than usual.
Babies are born with an immature immune system, dependent for their first few months on protection from mom. How the RSV vaccination will work: A single injection late in pregnancy gives enough time for the mom-to-be to develop virus-fighting antibodies that pass through the placenta to the fetus — ready to work at birth.
It’s the same way pregnant women pass along protection against other infections. Pregnant women have long been urged to get a flu shot and a whooping cough vaccine — and more recently, COVID-19 vaccination.
Pfizer’s study included nearly 7,400 pregnant women plus their babies. Maternal vaccination didn’t prevent mild RSV infection — but it proved 82% effective at preventing a severe case during babies’ first three months of life. At age 6 months, it still was proving 69% effective against severe illness.
Vaccine reactions were mostly injection-site pain and fatigue. In the study, there was a slight difference in premature birth — just a few weeks early — between vaccinated moms and those given a dummy shot, something Pfizer has said was due to chance. The FDA said to avoid the possibility, the vaccine should be given only between 32 weeks and 36 weeks of pregnancy, a few weeks later than during the clinical trial.
If enough pregnant women get vaccinated, Pfizer has predicted the U.S. could prevent as many as 20,000 infant hospitalizations a year and 320,000 doctor visits.
The only other option to guard babies from RSV: Giving them lab-made antibodies. The FDA recently approved a new drug that’s the first one-dose version, recommended for all infants younger than 8 months before their first RSV season starts. Beyfortus, from Sanofi and AstraZeneca, is expected to be available this fall.
Cincinnati’s Schlaudecker, a pediatric infectious disease specialist, said both the new antibody drug and the maternal vaccine are eagerly anticipated, and predicted doctors will try a combination to provide the best protection for babies depending on their age and risk during RSV season.
Another Cincinnati Children’s physician who’s cared for seriously ill RSV patients volunteered to participate in Pfizer’s vaccine study when she became pregnant.
“The last thing a parent wants to see is their kid struggling to breathe,” Dr. Maria Deza Leon said. “I was also at risk of being the person that could get RSV and give it to my son without even realizing.”
Deza Leon received her shot in late January 2022 and her son Joaquin was born the following month. While she hasn’t yet learned if she received the vaccine or a dummy shot, Joaquin now is a healthy toddler who’s never been diagnosed with RSV.
Specialty Care Referrals in NC Medicaid Reminder: NC Medicaid does not require referrals for specialty care.
This bulletin provides information about referrals for specialty care for NC Medicaid beneficiaries.
This bulletin provides information about referrals for specialty care for NC Medicaid beneficiaries. The information in this bulletin applies to both NC Medicaid Direct and NC Medicaid Managed Care.
Historically, in NC Medicaid, members were enrolled in Carolina Access (CA) and assigned a primary care provider (PCP). Additionally, in the past specialists were required to obtain a PCP referral and their CA provider number on the claim for the claim to process.
In November 2016, NC Medicaid removed the requirement to enter the CA provider number on the claim for claims adjudication. Additionally, providers were no longer required to enter referrals into NCTracks.
For patient care coordination purposes, specialist providers are encouraged to coordinate care and share records with the PCP when they are treating a member. However, since 2016, specialists do not need a referral from the PCP when deciding whether to treat a member with NC Medicaid coverage.
Although some specialty offices may require a PCP referral prior to treating a member, NC Medicaid and NC Medicaid Managed Care plans do not require a PCP referral prior to claims payment for specialist office visits.
- For NC Medicaid Direct members, there are no barriers for specialists to provide office visits (evaluation and management services) to members when the provider is enrolled with NC Medicaid.
- For members enrolled with NC Medicaid Managed Care plans, there are no barriers for specialists to provide office visits (evaluation and management services) to members when the specialist provider is enrolled with NC Medicaid and contracted with the members’ assigned health plan.
- If the member is enrolled with a NC Medicaid Managed Care plan and the provider is not in the members’ assigned health plan’s network (i.e., out of network with the plan), prior authorization may be required. For this reason, all out of network (OON) providers (primary care and specialists) should check with the members’ assigned health plan prior to seeing the patient.
- Providers should consult with the NC Medicaid Managed Care health plan to assure that any needed prior authorizations for certain tests or imaging services are obtained prior to rendering these services.
Plan Resources for Referrals and OON Requirements:
- AmeriHealth Caritas Provider Manual
- See Section V. Utilization Management > Referrals
- For OON:
- See Section V. Utilization Management > Prior Authorization Policy and Procedure
- See Section XI. Claims Submission Protocols and Standards > Claims Submission
- See Section XI. Claims Submission Protocols and Standards > General Procedures for Claim Submission
- Carolina Complete Provider Manual
- See Guidelines for Providers > Referrals (this contains information on OON providers)
- See Guidelines for Providers > Specialist Responsibilities
- Healthy Blue Provider Manual
- See Section 2.9 Role and Responsibilities of Specialty Care Providers
- See Section 5.6 Prior authorization/Notification Coverage Guidelines > Out-of-area/ out-of-network care
- United Healthcare
- PA for OON Requirements
- See General information
- Provider Manual
- See Chapter 2: Care Provider Standards and Policies > Specialist responsibilities
- See Chapter 4: Medical Management > Referral guidelines
- PA for OON Requirements
- WellCare Provider Manual
- See Section 4: Utilization Management (UM), Care Management (CM) and Disease managed (DM) > Utilization Management > Utilization Management Process > Referrals
- See Section 4: Utilization Management (UM), Care Management (CM) and Disease managed (DM) > Utilization Management > Authorization Request Forms
Summary
For NC Medicaid Direct and NC Medicaid Managed Care health plans there is no requirement for a specialist to require a referral from the PCP for NC Medicaid members.
- NC Medicaid Direct and NC Medicaid Managed Care do not require PCP referrals to see specialists, some specialists may require a referral from the PCP to see the patient.
- NC Medicaid and the NC Medicaid Managed Care health plans encourage coordination of care between specialists and the member’s PCP.
Provider Reverification Reminder
As a reminder, re-credentialing/reverification is an evaluation of a provider’s ongoing eligibility for continued participation in NC Medicaid, normally conducted every five years as mandated 42 CFR 455.414. Reverification is required for continued participation with NC Medicaid programs.
Now that the federal PHE has ended, NC Medicaid must ensure that all enrolled providers, including those whose reverification was delayed, are compliant with the federal regulation. Providers for whom reverification was delayed have been organized into groups to ensure the timely completion of the reverification process. For more information on provider reverification, see the Provider Reverification Reminder bulletin from June 20, 2023.
Contact: NCTracks Call Center: 800-688-6696
New Report: NC Hospitals Suing Thousands of Patients

(NPR, Noah Levey) -- North Carolina hospitals — led by the state's largest public medical system — have sued thousands of their patients since 2017, according to a new analysis that sheds additional light on the aggressive tactics U.S. hospitals routinely use to collect from people who fall behind on their bills.
The report, produced by the state treasurer and Duke University School of Law researchers, and related patient interviews offer harrowing accounts of people pursued for tens of thousands of dollars and often surprised by liens that hospitals placed on family homes.
In some cases, spouses were targeted after their partners died. In others, patients interviewed by researchers said they'd been surprised to learn about property liens only after they tried to sell their homes or after a parent who owned the home died.
"I know my house will never be mine. It is going to be the hospital's," said Donna Lindabury, 70, whose home was targeted by Charlotte-based Atrium Health, which won a $192,000 judgment against her and her 79-year-old husband over his 2009 heart surgery. Interest on the debt represented more than half of the couple's balance.
Lindabury said the hospital originally told them they could get assistance with the bills, but then denied their applications for aid. "People, where their God is money, they just don't care," she told researchers.
The North Carolina findings reinforce an investigation by KFF Health News and NPR, which found that most U.S. hospitals maintain policies to aggressively pursue patients for unpaid bills, using tactics such as lawsuits, selling patient accounts to debt buyers, and reporting patients to credit rating agencies.
Nationwide, about 100 million people — 41% of adults — have some form of health care debt, according to a KFF poll. Medical debt is most widespread in the South, where chronic disease is more prevalent and many states haven't expanded their Medicaid safety net through the Affordable Care Act. (North Carolina only expanded Medicaid this year.)
"Profits ahead of patients"
The North Carolina state treasurer released the new report as a growing number of states, including North Carolina, are working to expand protections for patients, often in the face of hospital industry lobbying.
"It's just another example of hospitals putting profits ahead of patients. It's like an onion. The more you peel it back, the more you cry," said Treasurer Dale Folwell, a Republican who for years has challenged hospital pricing and debt collection practices. "They should stop breaking people's kneecaps to collect these debts."
Atrium and other large tax-exempt health systems are under scrutiny amid mounting evidence that many aren't providing adequate financial assistance to low-income patients and are leaving people who should qualify for aid with big bills.
The new report, based on an analysis of 5½ years of court records from 2017 to 2022, identified 5,922 debt collection lawsuits that targeted more than 7,500 patients and their family members.
The suits generated more than $57 million in judgments for the hospitals, researchers found, including millions of dollars in interest charges and other fees assessed against patients and their families.
North Carolina law allows hospitals to charge 8% annual interest on outstanding debts, which added tens of thousands of dollars to some families' debts over the years, the researchers found. Overall, interest accounted for almost a third of the total judgments recorded in the debt cases.
The report also noted that the lawsuits undermine the financial security of generations of North Carolinians. Hospitals can pursue family members for a patient's medical debt, and property liens sap the value of a home, even after a patient dies.
"These lawsuits can thus target a family's primary source of equity for surviving spouses and children," the authors wrote. "Medical debt can fuel an intergenerational cycle of poverty."
Two hospitals file the most lawsuits
Researchers found that the most aggressive debt collector was Atrium, a medical system with roots as a public hospital in Charlotte that, following a merger last year with Midwest-based Advocate Aurora, is now a multistate colossus with $27 billion in annual revenue. Atrium filed almost 2,500 lawsuits against patients from Jan. 1, 2017, to June 30, 2022.
Atrium also pushes patients who can't afford medical bills into loans from private equity-backed lender AccessOne that can come with interest rates as high as 13%, an NPR and KFF Health News investigation found last year.
Atrium declined to address questions about the lawsuits on the record or to make chief executive Eugene Woods available to discuss its debt collection practices.
The second-most litigious system is much smaller. CaroMont Health in Gastonia, North Carolina, a small city about 20 miles west of Charlotte, operates just one inpatient hospital. But it filed almost 1,800 lawsuits against patients from 2017 to mid-2022, according to the report.
CaroMont declined to make chief executive Chris Peek available for an interview, but a spokesperson said the system only rarely sues. "We take seriously our obligation to partner with patients in all aspects of medical care and service, and we always try to resolve these matters with compassion," Meghan Berney said in a statement.
In contrast to Atrium and CaroMont, some North Carolina hospitals filed only one or two lawsuits against their patients from 2017 to 2022, the researchers, led by Duke law professor Barak Richman, found.
Hospitals suing patients is a nationwide pattern
Similar analyses of court records in Wisconsin, New York, Maryland, and other states in recent years have uncovered extensive use of the court system by hospitals. And KFF Health News found last year that more than two-thirds of U.S. hospitals sue patients or take other legal action against them, such as garnishing wages or placing liens on property. That analysis was based on an investigation of a sample of more than 500 hospitals nationwide.
The attention on these debt collection activities has helped catalyze state efforts to expand protections for patients. Several states, including Arizona, Colorado, Maryland, and New York, have enacted medical debt laws in recent years.
In North Carolina, a bipartisan group of state lawmakers have been pushing legislation that would restrict some collection activities by hospitals, including capping interest rates that medical providers could charge on patient debt and limiting collections against family members. Earlier this year, the state Senate unanimously passed the bill, called the Medical Debt De-Weaponization Act.
But the bill has stalled in the House amid opposition from the state's powerful hospital industry, whose political action committee has made more than $260,000 in campaign contributions since 2022, according to WBTV, the CBS affiliate in Charlotte.
Among the biggest beneficiaries of hospital industry largesse is the speaker of the North Carolina House, Republican Tim Moore, the station reported. Moore's office did not respond to inquiries from KFF Health News.
If you would like to respond to this article in the NCMS On Point blog, please contact Randy Aldridge at [email protected].
Physicians Advocacy Institute Releases Regulatory Advocacy Update

This newsletter outlines key federal regulatory developments and highlights PAI’s advocacy on matters that impact physicians and patients, including:
- Centers for Medicare and Medicaid Services (CMS) Suspends Surprise Billing Independent Dispute Resolution (IDR) Process
- CMS Releases Calendar Year (CY) 2024 Medicare Physician Fee Schedule (MPFS) and Quality Payment Program (QPP) Proposed Rule
- House Committee on Energy and Commerce Holds Hearing on Medicare Access and CHIP Reauthorization Act of 2015 (MACRA)’s Implementation and Remaining Challenges
- Bipartisan Lawmakers Send Letter to CMS on Improving Prior Authorization Processes
- CMS Announces Multi-State Initiative to Strengthen Primary Care
- Center for Medicare & Medicaid Innovation (CMMI) Publishes Strategy to Support High-Quality Primary Care
- Government Accountability Office (GAO) Releases Study on Non-Compete Agreements
- CMS Unwinds Mandatory COVID-19 Vaccination Requirement for Physicians
For information on PAI’s advocacy initiatives, physician payment resources and research, please visit PAI’s website. Healthsperien’s Resource Updates page also has information on key issues in health policy and identifies potential reforms under the Biden Administration, Congress and in the states. Additionally, CMS releases its QPP Small Practices Newsletter, a monthly resource that provides small practices (15 or fewer physicians) with program updates, upcoming QPP milestones and resources to support continued participation and success in the QPP. You can sign up here to receive this monthly resource
Top News & Wins
- CMS suspended the IDR process due to a federal district court ruling in favor of the Texas Medical Association (TMA) to vacate key provisions in CMS’ surprise billing regulations. PAI is pleased with the decision of the court and urges the Departments of Health and Human Services (HHS), Labor and Treasury (collectively, the Departments) to issue additional guidance promptly.
- CMS released its CY 2024 MPFS and QPP Proposed Rule, which is expected to reduce physician reimbursement by 3.36% in 2024. PAI strongly opposes any cuts to physician payments and will submit detailed comments arguing for updates to physician payment that reflect inflationary pressures increasing the cost of providing services.
- The House Committee on Energy and Commerce held an important hearing examining MACRA’s implementation along with the ongoing challenges it poses for physicians and patients.
CMS released the Making Care Primary model, which will provide participants with additional revenue to build infrastructure, make primary care services more accessible and better coordinate care with specialists.
CMS Suspends Surprise Billing IDR Process
On August 3, CMS suspended the IDR process established under the No Surprises Act to reconcile surprise billing payment disputes. CMS based this suspension on a recent ruling that vacated CMS’ December 2022 IDR fee increase and sections from the October 2021 interim final rule on claims batching. The court ruled in favor of TMA, finding that the Administration violated the Administrative Procedures Act with its 600% fee increase and rules on claims batching. CMS noted the Departments have temporarily suspended the federal IDR process, including the ability to initiate new disputes until the Departments can provide additional instructions. CMS has not indicated how long the portal may be suspended.
PAI supports the Court’s ruling, which is the third decision siding with TMA in litigation challenging federal regulations implementing the No Surprises Act. The fee increase from $50 to $350 dramatically curtailed many physicians’ ability to seek arbitration when a health plan offers insufficient payment for out-of-network care. PAI urges CMS to issue additional guidance swiftly to prevent further confusion and harm for physicians.
CMS Releases CY 2024 MPFS and QPP Proposed Rule
On July 13, CMS released (fact sheet here) its CY 2024 MPFS and QPP Proposed Rule. The proposed CY 2024 MPFS conversion factor is $32.75, a decrease of $1.14, or 3.36%, from the CY 2023 conversion factor of $33.89.
As medical inflation continues to add to the costs of providing services, as reflected by the 4.5% Medicare Economic Index, this proposed reduction in physician reimbursement is unsustainable. This misguided payment policy has driven physicians to sell or close their private medical practices to hospitals and other corporate entities like private equity firms. Additionally, small and independent physician practices are continuing to adjust to the wide-reaching and universally detrimental effects of COVID-19. Studies show consolidation diminishes competition and results in higher costs.
PAI strongly opposes any cuts to physician payments as practices cannot continue to absorb the increasing costs of providing services while their payment rates dwindle. A permanent solution is needed to fix this unsustainable payment system and end the need for Congress to “fix” statutory payment cuts each year.
PAI will continue to review key proposals to ensure they support the ability of physicians to sustain their medical practices. PAI will submit detailed comments on this proposal. Healthsperien released a comprehensive summary on this proposed rule which can be found here. Key provisions include:

House Committee on Energy and Commerce Holds Hearing on MACRA's Implementation and Remaining Challenges
On June 22, the House Energy and Commerce Committee’s Oversight and Investigations Subcommittee held a hearing examining MACRA’s implementation and ongoing challenges it poses for physicians and patients. Chairman Griffith (R-VA) provided opening remarks explaining MACRA has provided many benefits since its enactment in 2015, like eliminating the sustainable growth rate model and transitioning from a fee-for-service system to that of value-based care through MIPS and APMs. The hearing covered concerns about current reporting requirements and quality measures, with members calling requirements bureaucratic and overly burdensome, especially for small and rural practices. Several members raised the issue of inadequate physician reimbursement amidst physician shortages and advocated for legislation implementing an annual inflation-based update.
The hearing featured witness testimony from individuals representing the following organizations:
- Paragon Institute
- National Association of ACOs
- America’s Physician Groups
- Austin Regional Clinic in Texas
- Warren Alpert Foundation Professor of Health Care
- Harvard Medical School Department of Health Care Policy
Healthsperien attended the hearing and full notes are available to view here.
PAI consistently advocates for fair payment policies and rates for all physicians, and strongly opposes the continued budget neutrality rules that force a “zero sum” approach to physician payment. This approach fails to reflect the realities of physician practice expenses and pits certain physician specialties against others. Budget neutrality creates a system of “winners” and “losers” when reimbursement rates under the MPFS are adjusted. It also exacerbates an environment of uncertainty for independent practicing physicians who must respond to reduced reimbursement while maintaining their commitment to providing quality medically necessary care. As evidenced by PAI-Avalere research documenting consolidation trends, these pressures continue to drive physicians away from private practice and into employed arrangements.
Bipartisan Lawmakers Send Letter to CMS on Improving Prior Authorization Processes
On June 21, more than 230 House members and 61 Senators sent a letter to CMS requesting changes to a December 2022 proposed rule that streamlines the prior authorization process for insurers and physicians. The bipartisan group of lawmakers are led by Rep. Susan DelBene (D-WA) and request CMS to promptly finalize the rule and include provisions from DelBene’s Improving Seniors’ Timely Access to Care Act to improve the prior authorization process for Medicare Advantage (MA) plans. The letter asks CMS to establish a mechanism for real-time electronic prior authorization decisions for routinely approved items and services, require that plans respond to prior authorization requests within 24 hours for urgent care, and require detailed transparency metrics.
The letter indicates that both the legislation and CMS’ recent proposals would:

Earlier this year, PAI submitted a comment letter in response to CMS’ 2022 proposed rule with recommendations reflected in this Congressional letter. PAI strongly urged CMS to further enhance the rule’s impact through additional provisions to support adoption by physicians and strengthen the rule’s transparency requirements, including requiring transparency around the specific clinical guidelines used in prior authorization determinations. PAI also recommended additional reporting by payers on metrics that will inform efforts to further streamline and speed prior authorization processes (e.g., through “real time” determinations where appropriate) and protect patients from unnecessary delays in care.
CMS Announces Multi-State Initiative to Strengthen Primary Care
On June 8, CMS announced the Making Care Primary (MCP) Model, which will be tested under CMMI in eight states—Colorado, Massachusetts, Minnesota, New Jersey, New Mexico, New York, North Carolina and Washington. The MCP Model will run for 10 and a half years, from July 1, 2024, to December 31, 2034, and will provide participants with additional revenue to build infrastructure, make primary care services more accessible and better coordinate care with specialists. The primary goals of the MCP are to:

CMS is working with Medicaid Agencies in the participating states to engage in full care transformation across public programs, with plans to engage private payers in the coming months. The model includes a progressive three-track approach based on participants’ experience level with value-based care and APMs. Track One will focus on building infrastructure to support care transformation. In Tracks Two and Three, the model will include certain advance payments and offer more opportunities for bonus payments based on participant performance.
PAI commends CMS for considering the needs of small and rural practices interested in engaging in value-based arrangements (VBAs). Physician participation in VBAs requires considerable administrative support–VBAs are complex and expose physicians and their practices to financial risk and other challenges. PAI developed a comprehensive resource for members on APM financial methodologies, attribution, alignment and benchmark calculation, and other important factors to consider when participating in models, which can be accessed here.
CMMI Publishes Strategy to Support High-Quality Primary Care
On June 9, CMMI released its strategy to support high-quality primary care. The strategy aims to strengthen primary care infrastructure in the United States by creating multiple pathways to support improved financing for advanced primary care, equitable access to high-quality primary care and sustainable transformation among a heterogeneity of practices.
The approach focuses on the following three dimensions:

The publication discusses current work addressing these areas, including Making Care Primary, the newest CMMI primary care model. CMMI is also testing a new alignment strategy promoting payer partnership, alignment on key model design features and encouraging payers to innovate for their unique patient population. The strategy also outlines future pathways to addressing the three focus areas. These efforts include exploring ACO-based primary care model tests that may focus on practices in the MSSP and exploring a state-based, total cost-of-care model.
GAO Releases Study on Non-Compete Agreements
On May 11, the GAO released a new report (one-pager) requested by Senators Chris Murphy (D-CT), Todd Young (R-IN), Elizabeth Warren (D-MA), Marco Rubio (R-FL), Ron Wyden (D-OR) and Tim Kaine (D-VA) on non-compete agreements (NCAs). The report found the use of NCAs is widespread throughout the U.S. labor market and serves to protect the interests of businesses while restricting job mobility, lowering wages for workers and discouraging innovation. Fifty-seven percent of health care and social assistance employers surveyed reported requiring new hires to sign agreements that restrict the workers from taking jobs with competing organizations. The reasons health care employers cited for requiring noncompete agreements included: to protect trade secrets, intellectual property and proprietary information; to prevent departing workers from taking clients with them; and to retain trained staff. The Federal Trade Commission proposed rule to ban NCAs is currently pending finalization and is expected to be released early next year.
PAI recognizes that the inclusion of non-compete clauses in contracts can prevent physicians from practicing medicine in their communities when they want to change jobs—limiting patients’ access to their physicians. PAI believes that federal guidance should be kept to a minimum and encourage states to adopt a few core principles for non-competes. PAI believes that non-competes should not be crafted in a way that infringes upon continuity of care.
CMS Unwinds Mandatory COVID-19 Vaccination Requirement for Physicians
On May 31, CMS published a final rule withdrawing COVID-19 vaccination requirements for certain workers at CMS-covered health care facilities. On November 5, 2021, the Biden Administration and CMS issued an interim final rule (IFC) which required Medicare- and Medicaid-certified physicians to be vaccinated against COVID-19. CMS recognizes that vaccines are important for preventing severe illnesses and promoting public health. However, as the incidence of severe COVID-19 has declined significantly since the IFC was issued, CMS will withdraw the health care staff COVID-19 vaccination provisions. CMS still strongly encourages facilities, when the opportunity exists and resources allow, to facilitate the vaccination and education of all individuals who provide services infrequently or frequently.
On The Lighter Side – August 11, 2023 - Honey, Golf, Jason Aldean, the Motel Keys, and a Pink Cheeseburger!
Here are some things NCMS employees, members, and YOU are talking about this week!
48th Annual Waldensian Festival in Valdese
The Festival Saturday includes a lineup of day-long entertainment at Main Stage, from theatrical performances to gravity-defying juggling acts. Over 140 specialty vendors will line Main Street. The Festival Finale will begin at 7:00 P.M. as country sensation, Ryan Perry takes the stage. All you need to know is here.
Sweet Flavors at Sourwood Festival in Black Mountain

Voted #1 Festival in Western North Carolina for 2022! Savor the sweet flavor of Sourwood honey, ice cream, food, arts, and crafts, two music stages, pony rides, a petting zoo, and craft demonstrations make for a day of family-friendly fun. Saturday and Sunday in Black Mountain.
2023 FedEx St. Jude Championship

World No. 1 Scottie Scheffler begins the 2023 FedEx Cup Playoffs attempting to win the title that barely eluded him last season. Memphis will see most of the tour's top players, including last years winner Rory McIlroy. This year, Scheffler enters the first leg of the playoffs ranked No. 2 in the FedEx Cup standings, behind only Jon Rahm. Scheffler is the +600 favorite in the 2023 FedEx St. Jude Championship odds. Click here for how to watch.
Summer Concerts Continue!

Tonight
- Jason Aldean, Coastal Credit Union Music Pavilion, Raleigh
- Ying Yang Twins, Cone Denim Entertainment Center, Greensboro
- Boney James, Koka Booth Amphitheater, Cary
Saturday
- Big Something, Greenfield Lake Amphitheater, Wilmington
- Grace Potter with Morgan Wade, Beech Mountain Ski Resort, Beech Mountain
- Rufus Du Sol, PNC Music Pavilion, Raleigh
- The Motel Keys, Treyburn Country Club, Durham
Sunday
- Tyler Childers, Red Hat Amphitheater, Raleigh
- Boney James, Knight Theater, Charlotte
- Patti Labelle, Johnny Mercer Theater, Savannah, GA
- Brit Floyd, Wilson Center, Wilmington
NCMB's Own The Motel Keys in Concert

All you need to know about the band and concerts is here.
North Carolina City Makes List For Most Pet Friendly City!

WalletHub’s annual list of the Most Pet-Friendly Cities in the U.S. has just come out! Raleigh is in the top 10 and Greensboro is first on the list for most budget friendly for dog owners!
Top 10 Pet-Friendly Cities in 2023
- Scottsdale, Arizona
- Tampa, Florida
- St. Petersburg, Florida
- Las Vegas, Nevada
- Colorado Springs, Colorado
- Birmingham, Alabama
- Atlanta, Georgia
- Raleigh, North Carolina
- St. Louis, Missouri
- Portland, Oregon
Barbie is Officially Everywhere! (would you eat this?)

Burger King introduces the BK Barbie Combo. It includes a cheeseburger topped with bacon bits and dressed with a “smoky” bright pink sauce. Check your local Burger King to try it for yourself!
If you have an event you would like added to On The Lighter Side, email Randy Aldridge at [email protected].
Dr. Karen L. Smith Honored with 2023 NC Rural Leadership Award
NCMS Board member Dr. Karen L. Smith received the 2023 Larry Wooten Rural Leadership Award, presented by Governor Roy Cooper and Agriculture Commissioner Steve Troxler at the North Carolina Executive Mansion.

RALEIGH -- Dr. Karen L. Smith and L.T. Ward have received the 2023 Larry Wooten Rural Leadership Award, presented by Governor Roy Cooper and Agriculture Commissioner Steve Troxler at a reception Wednesday at the North Carolina Executive Mansion.
Smith is a North Carolina Medical Society Board member and has been actively involved in membership, advisory roles, the Kanof Institute for Physician Leaders, and various committees. She has been a physician in private practice in Hoke County for more than 30 years. Smith has worked tirelessly to improve access to quality healthcare services for residents of rural communities including efforts to prevent diabetes, overcome COVID-19 vaccination hesitancy, and expand telehealth. Her pioneering work to implement innovative healthcare programs and advocate for policies that address the unique healthcare challenges faced by rural areas have made a profound difference in the lives of countless individuals. Smith has served in numerous leadership roles including as president of the North Carolina Academy of Family Physicians, the North Carolina Medical Society Board of Directors, and as an elected board member of the American Academy of Family Physicians. She has been recognized for her contributions with numerous awards including being named the 2016 North Carolina Family Physician of the Year and the 2017 American Academy Family Physician of the Year.
“Dr. Karen L. Smith's commitment to advancing rural health in North Carolina is truly commendable,” said Governor Roy Cooper. “Her dedication to ensuring that all North Carolina residents have access to quality health care has made a significant impact on rural communities. We are proud to honor Dr. Smith along with agricultural leader L.T. Ward with the Larry Wooten Rural Leadership Award for their exceptional contributions.”
L.T. Ward’s remarkable work in Western North Carolina agriculture has been instrumental in empowering farmers and fostering sustainable agricultural practices. As a passionate advocate for agriculture and rural development, Ward has played a pivotal role in driving economic growth and revitalizing rural communities through his innovative approach to farming.
The Larry Wooten Rural Leadership Award, established in 2019, celebrates outstanding leaders who have made a significant impact on North Carolina's rural communities. Named after its inaugural recipient, Larry Wooten, the award recognizes individuals who exemplify a deep commitment to rural endeavors, create lasting improvements in the lives of rural residents, and inspire others through their character and accomplishments.
About the Larry Wooten Rural Leadership Award
The Larry Wooten Rural Leadership Award was established in 2019 to honor exceptional leaders who have made significant contributions to rural communities in North Carolina. The award recognizes individuals whose visionary leadership and unwavering dedication have positively impacted the lives of those living in rural areas. The selection committee includes representatives from Hometown Strong (Governor Cooper's initiative for Rural North Carolina), the N.C. Department of Natural and Cultural Resources, and the N.C. Department of Agriculture. For more information, visit https://hometownstrong.nc.gov/larry-wooten-rural-leadership-award.
More about Dr. Smith
Karen Smith, MD, Named 2017 National Family Physician of the Year
NCMS Board Member Dr. Karen Smith Speaks Out on Healthcare Disparities
NCMS Members, VP Talk About AMA Collaboration on Diabetes Prevention
More photos of the event featuring Governor Roy Cooper, NCDHHS Secretary Kody Kinsley, Old North State Medical Society President Dr. Brian Shackleford, NCMS Chief Experience Officer Dr. Michelle Laws, and North Carolina Agriculture Commissioner Steve Troxler.
Work Smarter with HCLM Alum Dr. Jennie Byrne! New Webinar Helps You Master the Future of Work.

The North Carolina Medical Society is happy to announce a new webinar with Jennie Byrne, PhD, MD, DFAPA, a graduate of our KIPL Health Care Leadership and Management Program. Dr. Byrne will be discussing her book Work Smart: Use Your Brain and Behavior to Master the Future of Work.
Byrne graduated from HCLM in 2019 and has since continued her successful career path as an innovative and entrepreneurial leader. She is a long-time champion of the HCLM program and discusses her career in the NCMS Bulletin magazine. You can read her story here.
Byrne has an MD/PhD in Neurophysiology from New York University and did her psychiatry residency at Mt. Sinai Health System. She is also the Chief Patient Officer at Belong Health and is a sought-after speaker on several topics including efficiency, stress and well-being, leadership and more. She is a musician, wife ,and mother of two (and very busy!).
Her book, Work Smart, was published in 2023. There is also a most recent audio book version.
To join this session, register here.
To learn more about HCLM, click here.
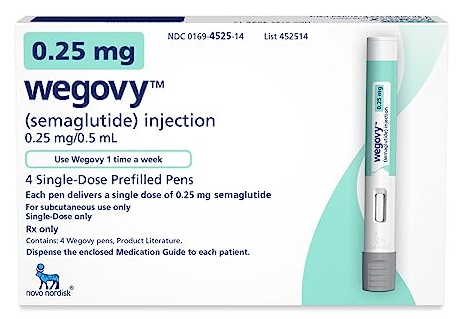
Wegovy Getting More Popular, Could Reduce Risk of Heart Attack and Stroke
Novo Nordisk Says Wegovy May Cut Risk of Cardiovascular deaths by 20 %

Novo Nordisk, maker of the wildly popular Wegovey, says the weight loss injection can cut risks of heart attacks, strokes, and cardiovascular deaths by 20 percent. This study is the first to show that obesity drugs can bring heart health to those who do not have diabetes. It may also add another spike of popularity to a drug that skyrocketed after others like Ozempic were used off-label for weight loss.
Novo Nordisk studied Wegovy against placebo in addition to standard of care for prevention of major adverse cardiac events in 17,604 adults with heart disease and obesity or who were overweight, but who didn’t have diabetes. It called the five-year trial “Select.”
The finding of a 20% reduction in heart risk is higher than many experts had anticipated. A similar trial for the type 2 diabetes drug Ozempic, which uses the same ingredient, semaglutide, previously showed it could reduce cardiovascular risk by 26% — but no trial had yet shown a risk reduction in people without diabetes.
Novo Nordisk’s executive vice president for development, Martin Holst Lange, said in the news release that the trial “has demonstrated that semaglutide 2.4mg has the potential to change how obesity is regarded and treated.”
The findings, once peer-reviewed and published in a medical journal, may encourage more doctors to prescribe the wildly popular weight loss drug, but could also potentially help improve insurance coverage.
Novo Nordisk said Wegovy appeared to have a “safe and well-tolerated profile” in the study, in line with what it has seen in previous trials.
The company said it will submit applications with regulators to add the cardiovascular benefits to the drug’s prescribing information, and said it will present detailed results from the trial at a scientific conference later this year.
Additional reading:
Wegovy shown to reduce risk of heart attack, stroke in major cardiovascular trial
Wegovy shown to cut risk of stroke and heart attacks, company says
Weight Loss Drug Cuts Risk of Heart Problems, Maker Says
Questions About New RSV Option for Infants? Pediatric Experts Answer Questions in Live Panel Discussion
You're invited to join the RSV National Expert Program to learn about a new FDA-approved option for infant RSV prevention August 16th at 7:00 p.m. ET and 7:00 p.m. PT


Join colleagues with questions surrounded newly approved RSV medication for infants.
To register for the online panel discussion click here.
Following the news of FDA-approval of Beyfortus™ (nirsevimab-alip) on July 17th, here are the ACIP recommendations the use of Beyfortus for all infants.
The ACIP recommendation for Beyfortus include1:
- Infants aged <8 months born during or entering their first RSV season are recommended to receive one dose of Beyfortus (50 mg for infants <5 kg and 100 mg for infants ≥5 kg)
- Children aged 8–19 months who are at increased risk of severe RSV disease and entering their second RSV season are recommended to receive one dose of Beyfortus (200 mg)
In addition to the recommendation, the CDC has voted to include Beyfortus in the Vaccines for Children (VFC) program.
- See the ACIP recommendation
- More information is also available in the US Sanofi Press Release
The Beyfortus ACIP recommendation was based on efficacy and safety results from Trial 03 (phase 2b study) and Trial 04 (phase 3 MELODY study). The populations from these clinical trials represented all infants, including healthy full-term infants, premature infants, and infants at higher risk for RSV infection.
INDICATION
Beyfortus is indicated for the prevention of respiratory syncytial virus (RSV) lower respiratory tract disease in:
- Neonates and infants born during or entering their first RSV season.
- Children up to 24 months of age who remain vulnerable to severe RSV disease through their second RSV season.
IMPORTANT SAFETY INFORMATION
Contraindication
BEYFORTUS is contraindicated in infants and children with a history of serious hypersensitivity reactions, including anaphylaxis, to nirsevimab-alip or to any of the excipients.
Please see additional Important Safety Information below.
Please see the full Prescribing Information for complete study design and results.
Beyfortus Immunization Codes for Billing and Reimbursement
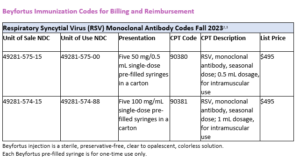
Click here to find more information on Beyfortus
Please see full Prescribing Information for details.
Chance discovery by Researchers Helps Fight Against Malaria
Scientists have found a naturally occurring strain of bacteria which can help stop the transmission of malaria from mosquitoes to humans.
(BBC News - Naomi Grimley) -- They found it by chance, after a colony of mosquitoes in one experiment did not develop the malaria parasite.
The researchers say the bacteria could be a new tool for fighting one of the world's oldest diseases, which kills 600,000 people every year.
Trials assessing its safety in the real world are now taking place.
"The infection rate in the mosquitoes started dwindling and so by the end of the year the mosquitoes just would not be infected with the malaria parasite," says Dr Janneth Rodrigues, who led the programme.
Further studies revealed that a specific strain of bacteria - TC1 - which is naturally present in the environment, had stopped the development of the malaria parasites in the gut of the mosquitoes.
"Once it colonises the mosquito, it lasts for the entire lifespan," says Dr Rodrigues.
"And we found out that, yes, it is the bacteria which was responsible for reducing transmission in those mosquitoes."
New data published in Science magazine suggests the bacteria can reduce a mosquito's parasite load by up to 73%.
The bacteria works by secreting a small molecule, known as harmane, which inhibits the early stages of the malaria parasite growing in the mosquito's gut.
This lays open the possibility of treating surfaces in areas where the insects rest with the active compound.
End the threat
More trials are now taking place at a contained field research facility called MosquitoSphere in Burkina Faso to assess how effective and safe it would be to use harmane at scale in the real world.
The hope is that by developing this bacteria-based intervention into a product, scientists may soon have another tool in the box against one of the world's oldest diseases.
Malaria kills about 620,000 people a year - often children under the age of five. Vaccines have now been developed, but they are still in the early stages of being rolled out in Africa.
Gareth Jenkins, from the charity Malaria No More, said the new discovery was promising.
"With a strong innovation pipeline, it is possible to end the threat of malaria in our lifetimes."
UNC School of Medicine Selects Residents for 2023 Cefalo Awards
Five Residents Selected to Receive 2023 Cefalo House Officer Awards

The 2023 awards were given to Michael Cunningham, MD, Alexander Fenn, MD, Calvin Gross, MD, Wesley Roten, MD, and Krishan Sivaraj, MD.
The Robert C. Cefalo, MD, House Officer Award recognizes members of the Hospitals’ House staff for exemplary service to patients and families, professional performance and compassionate patient care. The 2023 awards were given to Michael Cunningham, MD, Alexander Fenn, MD, Calvin Gross, MD, Wesley Roten, MD, and Krishan Sivaraj, MD.
This award is intended to honor Robert C. Cefalo, MD, PhD, professor emeritus of obstetrics and gynecology and assistant dean, head of the Office of Graduate Medical Education, Emeritus, UNC School of Medicine. Cefalo served with distinction for 25 years as director of the residency and fellowship programs at UNC Hospitals. He was also a North Carolina Medical Society member, NCMS Foundation donor, and member of the NC Obstetrical and Gynecological Society.
Cefalo passed away in 2008 after a battle with cancer, and this award was renamed the Robert C. Cefalo House Officer Award in 2008 as a permanent memorial to the legacy he left behind. A selection committee of physicians, nurses, and other clinical and administrative staff select honorees each year.
Residents and fellows are nominated for this esteemed award by demonstrating outstanding empathy for patients and families, effective listening to and communicating with patients and families, advocacy for and demonstration of the highest standards in patient care, and exemplary professional and interpersonal interactions with colleagues, staff, patients, and visitors. In addition to this recognition, awardees are also provided a $1,000 professional development stipend.
Nominations for Michael Cunningham, MD, explained:
” Mike is extremely kind, thoughtful, and brilliant when it comes to teaching residents, students, and patients. He makes the learning environment comfortable and productive with his calm demeanor and extensive knowledge base. He exemplifies what it means to be a great doctor, medical educator, and patient advocate.”
“Mike is the epitome of an exemplary house officer through exceptional service, professionalism, and compassionate patient care. Mike is the ultimate team player and has been since he came to UNC for Medical School nearly 10 years ago.”
Nominations for Alexander Fenn, MD, explained:
“In the emergency department (ED), Dr. Fenn is a mature, professional, compassionate, and
empathetic emergency physician. With his patients, he quickly establishes excellent rapport and provides above and beyond, compassionate care. He has a natural ability to help patients on their worst day. Many patients seek ED care due to severe illness or pain, worry, and lack of access to care. He presents himself in a calm and understanding demeanor to each and every patient. He is able to hear their concerns, reassure them, and treat their ailments.”
“Alex is a selfless leader who continually strives for excellence and improvement. He is a natural leader, and I have no doubt he will make great improvements to the UNC EM program over the next year, but more importantly, will emerge as a leader within the field of Emergency Medicine in general.”
Nominations for Calvin Gross, MD, explained:
“A few things really stand out to me about Calvin that make him such a special person and resident physician. One, he is incredibly thoughtful and reflective. In meetings, he is often the one to bring a new insight or perspective to an issue. Two, he is incredibly kind. He gives his time to his patients, students, and his co-residents. Three, he is brilliant. He obviously reads and actively works to expand upon his knowledge. And finally, his communication skills are as one faculty member described ‘aspirational.'”
“Dr. Gross was a fantastic team leader who did an exemplary job setting expectations, managing interns, and ensuring good communication across the care team. He took time to educate the medical students and did a wonderful job incorporating them into patient care. He completed the TARHEEL Residents as Educators program, and I could see how well he implemented the tools he developed for medical education while on service.”
Nominations for Wesley Roten, MD, explained:
“Dr. Roten has the personal characteristics that make him an exemplary physician. He is bright, motivated, and has a wonderful sense of humor. He has natural leadership skills and will serve as our chief resident next year. He has already shown his leadership gifts during the transition period in preparation for next year. He understands the many guidelines and procedures required in the residency program and balances those guidelines with the personal needs of individual residents, which is a sometimes-challenging task. He is committed to medical student education and is a great role model for students.”
“In medical school at UNC Dr. Roten was a Kenan Rural Scholar, with a commitment
to serving a rural and underserved community post-training. Furthermore, in residency, he committed to our FQHC track and in our 2nd class of residents who completed their continuity clinic at the Siler City Community Health Center, a federally qualified health center about 40 minutes from Chapel Hill serving a large Spanish speaking population.”
Nominations for Krishan Sivaraj, MD, explained:
“He has been not only an excellent physician and clinician, but has demonstrated outstanding professionalism, communication skills, teamwork, and leadership. He is noted by faculty to be hard working and motivated, ‘forward-thinking,’ and dedicated to his patients. One attending wrote that “despite the workload, Krishan came everyday with a positive attitude and thoughtful management.” In clinic, his preceptors have written that he is ‘one of the best residents I’ve ever worked with. He is kind and caring. Always prepared. Constantly improving his approach… Careful thinker who takes into account the context of his patient’s lives…. He is remarkably self-aware.'”
American College of Physicians Recognizes NC Chapter, NCMS Member Dr. Marion McCrary

Congratulations are in order for the North Carolina Chapter of the American College of Physicians. This year, ACP is recognizing NC,ACP with the John Tooker Evergreen Award!
North Carolina has been recognized for its work to promote physician well-being. Under the leadership of North Carolina Medical Society member, Marion McCrary, MD, FACP, the NC,ACP has been proactive in responding to the challenge of physician burnout by sharing resources, regularly posting Wellness Wednesday advice, and producing web-based education offerings.
Update: FDA approves first postpartum depression pill in the US

( CNN) — The US Food and Drug Administration has approved the medication zuranolone for the treatment of postpartum depression – making it the first FDA-approved oral pill in the United States specifically for postpartum depression, a serious mental illness that can develop in about 1 in 7 new mothers after childbirth.
On Friday, the FDA announced that the treatment, to be sold under the brand named Zurzuvae, has been approved as a once-daily pill taken over the course of 14 days.
“Postpartum depression is a serious and potentially life-threatening condition in which women experience sadness, guilt, worthlessness—even, in severe cases, thoughts of harming themselves or their child. And, because postpartum depression can disrupt the maternal-infant bond, it can also have consequences for the child’s physical and emotional development,” Dr. Tiffany R. Farchione, director of the Division of Psychiatry in the FDA’s Center for Drug Evaluation and Research said. “Having access to an oral medication will be a beneficial option for many of these women coping with extreme, and sometimes life-threatening, feelings.”
To read the full article click here.
Additional reading:
FDA Approves First Oral Treatment for Postpartum Depression
The FDA approves the first pill specifically intended to treat postpartum depression
New Trial Data: Postpartum Depression Pill Shows Positive Effects
NCDHHS Secretary Kody Kinsley to Donate Blood, Celebrating Expanded Eligibility for Donations
Kinsley Joined Effort by Nine States and the District of Columbia to Push For Change

NCDHHS -- The American Red Cross begins accepting donations Monday, Aug. 7, from newly eligible individuals, many in the LGBTQ+ community, under the U.S. Food and Drug Administration expanded eligibility recommendations. North Carolina Department of Health and Human Services Secretary Kody H. Kinsley will be donating blood under these rules in Raleigh on Monday. State Health Director and NCDHHS Chief Medical Officer Elizabeth Cuervo Tilson, M.D., will also be donating blood.
Secretary Kinsley and Dr. Tilson led an effort, joined by nine other states and the District of Columbia, calling on the FDA to update this policy.
The American Red Cross collects, processes and distributes about 40% of the nation’s blood supply, and with the constant need for donations, Secretary Kinsley and Dr. Tilson encourage all North Carolinians, and those newly eligible to donate, to be regular blood donors. NCDHHS is partnering with EqualityNC, Harmony and the LGBT Center of Raleigh to invite those in Raleigh and surrounding areas to register for Monday’s event and give blood alongside them.
Adopting these new recommendations is the best way to ensure there is a safe and robust supply of blood. Blood must be donated from another person and cannot be manufactured. Donations help accident victims, people with blood disorders and cancer patients. Each donation can help save more than one life.
Those who wish to donate can register for Monday’s event at rdcrss.org/3sfzwf6 and use sponsor code: DonateNC. To find other dates and locations across the state, schedule an appointment to give blood or platelets by using the Red Cross Blood Donor App, visiting RedCrossBlood.org or calling 1-800-RED CROSS (1-800-733-2767).
SPECIAL NOTICE: NCMS Issues Initial Slate of Nominees

The North Carolina Medical Society (NCMS) Nominating and Leadership Development Committee met via Zoom on Saturday, June 3, 2023, to interview candidates and vote for the initial slate of nominees for officers, directors, and AMA delegates. Per NCMS Bylaws, Component Societies, Specialty Societies, and Sections may submit additional nominations for these positions between now and August 11, 2023. Please use this form to submit nominations. For questions on the nominating process, contact Evan Simmons.
The following slate of nominees was selected by the Nominating and Leadership Development Committee:
NCMS Board of Directors
- President Elect: John J. Meier, IV, MD
- Secretary-Treasurer: Tracy L. Eskra, MD
- Region 3 Representative: Karen L. Smith, MD
- Region 4 Representative: Martin Palmeri, MD
- At-Large Member: Bryant A. Murphy, MD
- At-Large Member: William G. Ferrell, MD
- At-Large Member: Ronald B. Laney, Jr., MD
NC American Medical Association Delegation
- AMA Delegate: Mary Ann Contogiannis, MD
- AMA Delegate: Justin B. Hurie, MD
Nominating and Leadership Development Committee
- NLDC Region 2: John B. Chiavetta, MD
- NLDC Region 2: Damian F. McHugh, MD
The Committee will reconvene to determine the eligibility of any additional nominees. The final slate of candidates will be published to members by September 22, 2023. Under the NCMS’ governance structure, online voting for the candidates will begin on September 28, 2023, and continue until midnight on October 12, 2023. Paper ballots also will be available to those who need them.
The following are the members of the NCMS Nominating and Leadership Development Committee:
- Chair Michael Utecht, MD
- President-Elect Eileen Raynor, MD
- Ex-Officio Arthur Apolinario, MD
- Board Representative Jugta Kahai, MD
- Board Representative Rachel Keever, MD
- Region 1 Representative Latonya Beatty, MD
- Region 1 Representative Chris Grubb, MD
- Region 1 Representative Joe Navejar, DO
- Region 2 Representative Justin Hurie, MD
- Region 2 Representative Ronnie Laney, MD
- Region 2 Representative Madji Namde, MD
- Region 3 Representative Labron Chambers, MD
- Region 3 Representative Peter Fenn, PA-C
- Region 3 Representative Jim Hill, PA-C
- Region 4 Representative Tracy Bell, PA-C
- Region 4 Representative Frank Melvin, MD
- Region 4 Representative MaryShell Zaffino, MD
Is There A Covid Summer Surge This Year?
Covid-19 has spiked every summer since 2020 — and this year’s no exception.
Cases and hospitalizations have risen since mid-July, according to the CDC.
Per the CDC, last month the U.S. saw its first bump in hospitalizations since the U.S. ended its Covid-19 public health emergency in May. From July 9 to 15, the number of patients admitted with the virus rose 10.3 percent; the following week, it was up 12 percent.
The number of positive cases has become harder to determine now that the CDC has stopped releasing its detailed reports, but one way to measure the spread is through wastewater analysis. According to recent tests, more Americans are being infected, with the highest increases in the Northeast and South. Walgreens, the pharmacy chain, has also reported that 42 percent of its tests were positive in the last week of July, compared to 29 percent in late June.
North Carolina hospitalizations for Covid-19 show a slight increase, but are no where close to last summers rates.
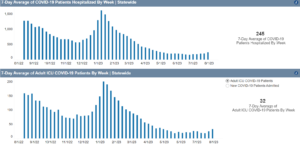
Why are Covid cases rising this summer?
As in previous years, it’s partly because the heat has forced more people to flock indoors and into the AC. The virus is more likely to get transmitted with more people mingling and breathing the same recirculated air. The rise in summer travel is also likely a factor, experts say.
Plus, for many of us, our immunity has begun to wane. Only 17 percent of Americans have received the bivalent vaccines, which were rolled out last fall and are supposed to protect against Omicron variants, Wired reports. Another reason for the uptick may be all the wildfire smoke this summer. There’s evidence that pollution may weaken the immune system — and in 2020, when California and the Pacific Northwest were subject to one particularly hellish season of smoggy air, the states saw an elevated level of Covid-19 fatalities, one study found.
Positive rates are “still near historic lows after 7 months of steady declines,” CDC spokesperson Kathleen Conley said last week. The number of fatalities has also reached an all-time low, The New York Times reports.
Leana Wen, M.D., a physician and professor of health policy and management at George Washington University, tells CNN that those who are generally healthy and have either been vaccinated or have been infected are not likely to become severely ill, but they still run the risk of developing long Covid. (It’s estimated that one in 10 Covid survivors end up with the condition.) Older adults and those with weakened immune systems are still vulnerable and Dr. Wen says they should avoid large indoor crowds, consider masking, and make sure they’re up-to-date on their boosters.
CDC: Preventive RSV Shot Recommended for ALL Infants
CDC: All Infants under 8 months of age should get a new antibody shot to protect RSV
 (CNN) -- All infants under 8 months of age should get a new antibody shot to protect against severe respiratory syncytial virus, or RSV, according a recommendation from the US Centers for Disease Control and Prevention.
(CNN) -- All infants under 8 months of age should get a new antibody shot to protect against severe respiratory syncytial virus, or RSV, according a recommendation from the US Centers for Disease Control and Prevention.
A panel of independent experts that advises the agency – the Advisory Committee on Immunization Practices, known as ACIP – voted unanimously on Thursday to recommend the injection, which will be added to the CDC’s childhood immunization schedule now that CDC director Dr. Mandy Cohen has signed off on the recommendation.
In a second unanimous vote, ACIP also recommended that certain infants ages 8 to 19 months get a second dose of nirsevimab to help them through their second RSV season, if they have underlying health issues that put them at higher risk for hospitalization.
“I’m very excited about this. I think this is going to be incredible,” said ACIP member Dr. Helen Talbot, a pediatrician at Vanderbilt University after the vote. “I think this is life-changing and I’m very excited. I just hope we can get through the hurdles,” to get it to patients, Talbot said.
RSV hits certain racial and ethnic groups harder than others. American Indian and Alaska Native children have hospitalization rates for RSV that are 4 to 10 times higher than the general population, so the CDC said they should be eligible to get a second shot, too.
Nirsevimab is a long-acting antibody — a protein that can recognize and handcuff RSV so it can’t infect cells — not a vaccine. It will be marketed under the trade name Beyfortus.
Committee members also voted to add the shot to the federally funded Vaccines for Children Program, which provides immunizations free-of-charge to children who might not get them otherwise.
“This new RSV immunization provides parents with a powerful tool to protect their children against the threat of RSV,” Cohen said in a statement. “RSV is the leading cause of hospitalizations for infants and older babies at higher risk and today we have taken an important step to make this life saving product available.”
The shot will be the first form of passive immunization added to the childhood immunization schedule.
In contrast to vaccines, which prompt the body to make antibodies against pathogens, passive immunizations don’t require the body to make anything. These therapies send protective antibodies into the body ready to go to work.
Political Pulse August 4, 2023. It Is Time for YOU to Get Involved in NCMS Advocacy!
As the long session winds down, it’s time for NCMS members to get to work! NCMS has four committees that deal with advocacy and you can be involved! Hannah Rice and Emma Kate Sowder break down the Legislative Cabinet, NCMS PAC Board, Policy Committee, and the Ethical & Judicial Committee. Enjoy our latest Political Pulse and then let us know how you would like to get involved.
https://youtu.be/lqqW45Qn2jA
Here is a quick and easy form to start your journey! Click here
Sign Up Now! 2023 AADA Legislative Conference


Join us on the Hill! Sign up today!
Registration is open for the AAD’s 2023 Legislative Conference. This annual event in Washington, DC offers a great opportunity to help dermatology advance its federal advocacy priorities. The NC Dermatology Association has participated in the conference for many years and we are looking forward to again have a delegation of North Carolina dermatologists attend.
The conference includes sessions on the latest legislative and regulatory activities impacting dermatology, with excellent national speakers, and is highlighted by a day on Capitol Hill with visits to our state’s Senate and House offices.
This year’s AAD Legislative Conference is scheduled for Sunday, September 10, through Tuesday, September 2 For more conference details, including registration and lodging information, go to: 2023 AADA Legislative Conference.
Come join your colleagues in the nation’s capital for an enlightening and enjoyable experience. Sign up today!
MIT-Designed Wearable Could Detect Breast Cancer Early and at Home

(Nice News) -- Fatma Caliskanoglu got regular breast cancer screenings, but still ended up developing the disease and dying just six months after her diagnosis. While sitting at her bedside, her niece Canan Dagdeviren started dreaming up a device that could prevent cases like her aunt’s — a device that could detect breast cancer in the time between mammograms.
Last week, that dream came to fruition when the MIT professor and a team of researchers published a study unveiling the Conformable Ultrasound Breast Patch. The wearable can attach to a bra and take ultrasound images at home that are comparable to those taken in medical settings. “It’s portable and easy to use, and provides real-time, user-friendly monitoring of breast tissue,” Dagdeviren said in a press release.
The technology could help spot interval cancer, breast tumors that form in between regularly scheduled screenings. Interval cancers account for 20%-30% of all breast cancer cases, and they tend to be harder to treat — the survival rate for patients with tumors detected in later stages is around 25%.
“My goal is to target the people who are most likely to develop interval cancer,” said Dagdeviren. “With more frequent screening, our goal [is] to increase the survival rate to up to 98%.”
Read the study here.
Click here to see how it works
New Study: AI Improves Breast Cancer Detection Rate by 20 Percent
Results from a study in Sweden show the potential of using artificial intelligence in mammography.
(Politico, Ashleigh Furlong) -- Artificial intelligence is able to accurately detect 20 percent more breast cancers from mammograms than traditional screening by radiologists, according to early results from a Swedish trial published overnight.
The study is the first randomized controlled trial to look at using AI in breast cancer screening and comes amid a dramatically shifting landscape for the technology and how it’s regulated.
The interim results, published in the Lancet Oncology late Tuesday, found that using AI-supported analysis of mammograms, alongside either one or two radiologists, was as good as using two radiologists without AI and led to 20 percent more cancers being detected.
There was also a significant reduction in workload for radiologists, with the doctors having to spend 44 percent less time reading mammograms.
The trial, which is still ongoing, was conducted in Sweden and looked at over 80,000 women. Half the participants had two radiologists look at their mammograms without AI, while the other half had their mammograms analyzed by AI and a radiographer — except in cases where AI generated the highest risk score, in which case two radiologists assessed the screening.
The results come amid a significant uptick in interest around the potential opportunities and risks that AI poses for medicine and the world more broadly. AI is increasingly being deployed in medical settings, but there is also concern about how algorithms are trained and validated in these spaces, as well as the potential for bias and over-diagnosis.
Meanwhile, the EU is planning stringent regulation around the use of AI and the European Medicines Agency is working on assessing the risks and benefits of the technology for drug development.
More to be done
Despite the positive findings, lead author Kristina Lång from Lund University in Sweden said the interim safety results “are not enough on their own to confirm that AI is ready to be implemented in mammography screening.” Lång and her colleagues are waiting for further results from the trial that will indicate whether using AI reduces the number of cancers detected between screenings and so if it is worth rolling out.
“The greatest potential of AI right now is that it could allow radiologists to be less burdened by the excessive amount of reading,” said Lång. She said AI could eliminate the need for a second radiologist to read the mammogram, allowing radiologists to help more patients.
Commenting on the results, Stephen Duffy, professor of cancer screening at Queen Mary University of London, said that reducing the burden on radiologists’ time was “an issue of considerable importance in many breast screening programmes.” But he said there could be concerns that using AI might over-detect harmless lesions.
As well as being the first randomized trial looking at using AI to help read mammograms, the study was one of the first to look at AI in the field of radiology. An editorial in European Radiology in January argued that randomized controlled trials were important for monitoring the safety of AI systems, which can produce “unpredictable and undetectable errors, not explainable by human logic.”
Additional Reading
AI-supported mammogram screening increases breast cancer detection by 20%, study finds
AI use in breast cancer screening as good as two radiologists, study finds
AI-Supported Breast Screens May Reduce Radiologist Workload
On The Lighter Side – August 4, 2023 - From Mt. Mitchell to Bald Head Island (plus Beyonce)!
Here are some things NCMS employees, members, and YOU are talking about this week!
OLD BALDY RUN FOR THE LIGHT - NATIONAL LIGHTHOUSE DAY FESTIVAL

Celebrate National Lighthouse Day with Old Baldy Run for the Light Race and festivities at North Carolina's oldest lighthouse, Old Baldy!
The Run for the Light course is a scenic run near coastal dunes and through the maritime forest on Bald Head Island on Sunday, August 6.
Information and registration is available here.
Mount Mitchell Arts & Crafts Fair

It is the first Friday and Saturday in August in historic downtown Burnsville and features more than 200 vendors and performers. It is the first Friday and Saturday in August in historic downtown Burnsville and features more than 200 vendors and performers.
See vendors and events here.
US Women's World Cup Continues. US vs. Sweden in Knockout Round on Sunday

It's win or go home for the U.S. women's national team as it enters the knockout rounds at the 2023 FIFA Women's World Cup on a quest to win third-straight title. The Americans would be the first team in FIFA history to capture three straight trophies, but they'll have a gauntlet of matches to survive. The action starts at 5am EST
For more commentary and how to watch click here.
Summer Concerts Continue!

Tonight
- Jason Mraz, Red Hat Amphitheater, Raleigh
- Cody Jinks, Coastal Credit Union Music Pavilion, Raleigh
- Shakey Graves, Rabbit, Rabbit, Asheville
Tomorrow
- Johnny Mathis, DPAC, Durham
- Jason Mraz, Skyla Credit Union Amphitheater, Charlotte
- Deana Carter, Rockingham Showcase, Reidsville
- Jessy J, Middle C Jazz, Charlotte
- Wayne Wonder, Red Hat Amphitheater, Raleigh
Sunday
- Snoop Dog, Coastal Credit Union Music Pavilion, Raleigh
- Dirty Heads, Roanoke Island Festival Park, Manteo
- Tyler Childers, Red Hat Amphitheater, Raleigh
- Bret Michaels, PNC Music Pavilion, Charlotte
- Beyonce, FedEx Field, North Englewood, MD
- Fortune Feimster, Harrah's Cherokee Center, Asheville
If you have an event you would like added to On The Lighter Side, email Randy Aldridge at [email protected].
Congressional Update - Action Needed to Fix Medicare. How You Can Help Now.
Congressional Update: Action Needed to Fix Medicare

The need for Congress to act quickly and decisively to fix a broken Medicare payment system cannot be overstated. The fiscal stability of physician practices and the long-term viability of our nation’s entire health care system hang in the balance.
The NC Medical Society has been engaged in the ongoing effort to reform the physician payment formula for many years, dating back to the national push to eliminate the antiquated Sustainable Growth Rate (SGR) which was in effect from 1997 until 2015. When the SGR was replaced by MACRA, there was universal acknowledgement that the transition was an improvement but not a permanent solution to the dilemma that physicians face annually in trying to keep pace with the rising cost of serving the Medicare population. NCMS efforts have included numerous trips to Capitol Hill, in-district meetings with our members of Congress, countless sign-on letters and calls, and a steadfast commitment to the collective effort among our partner organizations dedicated to Medicare reform.
The Society is presently working closely with our national and state partners to promote outreach to Congress, including the American Medical Association. Medicare payment reform is the first pillar of the AMA’s Recovery Plan for America’s Physicians and the AMA has developed and shared numerous resources that are helping our effort.
North Carolina saw a 24.5% increase in Medicare beneficiary enrollment from 2013 through 2021 - from 1.7M to 2.1M. The resulting access-to-care challenge that Medicare patients face will only get worse without reform of the current payment system.
Relief from annual funding cuts is an immediate need, along with fundamental changes to Medicare reimbursement centered on simplicity, predictability, relevance and alignment, as outlined in recommended reform principles. The current Medicare physician system does not reflect those principles, which we are working to change.
The government’s Medicare Economic Index (MEI), which gauges the inflation in medical practice costs, will hit 4.5% in 2024, its highest level in two decades. When combined with the 3.8% hike in the MEI recorded in 2023, that means medical practice costs increases will have exceeded 8 percent over just two years. Many individual and group physician practices have experienced cost increases well beyond this level, while still seeking a full recovery from pandemic-related setbacks. This dilemma has put patients ultimately in the crosshairs.
Adding to the pain, provisions in current law require the Centers for Medicare & Medicaid Services to implement a 2%, across-the-board reduction in Medicare physician payment rates this year, with another 3.36% cut to the conversion factor scheduled for 2024. In stark contrast to the annual payment increases tied to inflation given to hospitals, skilled nursing facilities and other entities that bill Medicare, physician practices do not qualify for automatic annual inflationary adjustments, requiring us to fight to reduce or delay payment cuts nearly every year.
Taking inflation into account, Medicare physician payment rates fell 26% from 2001 to 2023, while practice costs rose by 47% over the same period. We must place physician practices on sound financial ground through Congressional action to fix the Medicare physician payment system.
Legislative relief – An opportunity for you to get involved
The NC Medical Society joins the AMA and our many national specialty society partners in strongly supporting the Strengthening Medicare for Patients and Providers Act (H.R. 2474), a bipartisan measure now pending in the 118th Congress. This legislation would provide the crucial link between the Medicare physician payment schedule and the MEI, and finally put physicians on an equal fiscal footing with other entities drawing Medicare payment.
We are asking NCMS members to contact their members Congress through this AMA portal to urge passage of H.R. 2474.
When you reach out to those who represent you, in addition to expressing concern, also ask for a meeting, either in person or virtual, during their August recess or later this year. If you are able to set up a meeting, either with a House or Senate member or with one of their staff members, resources to help you are available at FixMedicareNow.org, including plain language explanations of the need for: automatic, annual inflation-based payment updates, reforms to budget neutrality policies, and simplifying the Merit-Based Incentive Payment System (MIPS). Every call and every meeting make a difference, especially when you share how the current Medicare payment system is impacting patients and your practice.
If you need help making a connection as a constituent, contact Alan Skipper, NCMS Vice President/External Affairs ([email protected]).
The North Carolina Medical Society will continue its fight for a financially stable and more predictable Medicare physician reimbursement model that protects your Medicare patients and you. We ask that you join us in urging Congress to ensure that Medicare continues to fulfill its crucial role in safeguarding both the health and financial well-being of tens of millions of Americans. Thanks for being an active advocate.
Congress Urges CMS to Fix Prior Authorization
 (AMA) -- A bipartisan majority from both chambers of Congress is pressing the Centers for Medicare & Medicaid Services (CMS) to finalize a pending federal regulation that would overhaul prior-authorization requirements within Medicare Advantage.
(AMA) -- A bipartisan majority from both chambers of Congress is pressing the Centers for Medicare & Medicaid Services (CMS) to finalize a pending federal regulation that would overhaul prior-authorization requirements within Medicare Advantage.
“We urge CMS to promptly finalize and implement these changes to increase transparency and improve the prior-authorization process for patients, providers and health plans,” wrote the lawmakers. “We are pleased that these proposed rules align with the bipartisan, bicameral Improving Seniors’ Timely Access to Care Act, which proposes a balanced approach to prior authorization in the [Medicare Advantage] program that would remove barriers to patients’ timely access to care and allow providers to spend more time treating patients and less time on paperwork.”
A bipartisan, bicameral letter to the Department of Health and Human Services (HHS) and CMS was anchored in the Senate by Sens. Sherrod Brown, D-Ohio, Roger Marshall, MD, R-Kan., Kyrsten Sinema, I-Ariz., and John Thune, R-S.D. Meanwhile, the effort in the House of Representatives was led by Reps. Suzan DelBene, D-Wash., Mike Kelly, R-Pa., Ami Bera, MD, D-Calif., and Larry Bucshon, MD, R-Ind. These same eight lawmakers introduced the Improving Seniors’ Timely Access to Care Act.
Ultimately, 61 Senators cosigned the letter, along with 233 House members. The AMA helped spearhead support for the letter. The AMA’s Physicians Grassroots Network and Patients Action Network worked to ensure a robust number of members of Congress cosigned this important communication to CMS.
The congressional majority also urged CMS to expand on the proposed rule by:
- Establishing a mechanism for real-time electronic prior authorization decisions for routinely approved items and services.
- Requiring that plans respond to prior-authorization requests within 24 hours for urgently needed care.
- Requiring detailed transparency metrics.
While all of these concepts are explored in the CMS proposed prior-authorization rule that was released last December, the letter provides the agency with an additional push from Congress to include these strengthened policies in the final regulation. The opportunity for outside stakeholders to provide feedback on proposed rule closed in March and AMA submitted detailed comments (PDF) that largely supported the vast majority of the concepts included in the proposed rule.
Why it’s important: While payers claim that prior-authorization requirements are used for cost and quality control, a vast majority of physicians report that the protocols lead to unnecessary waste and avoidable patient harm. One-third of the 1,001 physicians surveyed (PDF) by the AMA in December reported that prior authorization has led to a serious adverse event for a patient in their care.
More specifically, the AMA survey found that these shares of the physician respondents reported that prior authorization led to:
- A patient’s hospitalization—25%.
- A life-threatening event or one that required intervention to prevent permanent impairment or damage—19%.
- A patient’s disability or permanent bodily damage, congenital anomaly or birth defect, or death—9%.
The Improving Seniors’ Timely Access to Care Act would help fix prior authorization within Medicare Advantage, and the legislation was strongly supported by the AMA, which played a major role in securing enough co-sponsors to ensure the bill passed the House of Representatives (PDF) last September. The legislation, however, stalled in the Senate due to a flawed $16 billion cost estimate from the Congressional Budget Office (CBO).
The Biden administration’s pending prior-authorization regulation provides a key pathway for overcoming the high price tag associated with enacting the legislation. If the final prior-authorization regulation includes a mechanism for issuing real-time decisions, requirements to complete emergency requests within 24 hours, and detailed transparency metrics, then these policies must in turn be incorporated into CBO’s baseline estimate for the legislation.
That could mean a big drop in the agency’s $16 billion cost estimate for the legislation, thus upping the odds of congressional passage.
Learn more: Patients, physicians and employers can learn more about reform efforts and share personal experiences with prior authorization at FixPriorAuth.org.
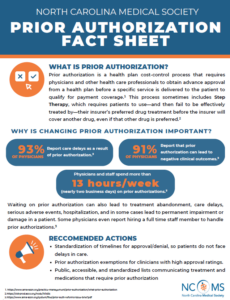
ADDITIONAL READING:
Bills in 30 states show momentum to fix prior authorization
Advocacy in action: Fixing Prior Authorization
1 in 3 doctors has seen prior auth lead to serious adverse event
Family of ‘Immortal’ Henrietta Lacks Reach Settlement with Thermo Fisher Scientific

(Portions of this story are from BBC News and Morning Brew) -- The family of Henrietta Lacks, whose cells underpin much of modern medicine, reached a settlement yesterday with biotech company Thermo Fisher Scientific.
Lacks’s descendants filed a lawsuit against Thermo Fisher in 2021, claiming that the company profited off of her cells for decades by selling her cells and seeking to obtain IP rights to medical advancements that they were used to develop.
Henrietta Lacks was a Black woman who died of cervical cancer in 1951. While receiving treatment at Johns Hopkins, her cells were harvested without her knowledge or consent. When researchers observed her cells in the lab, they found something surprising: They multiplied, whereas others would die within days. Scientists dubbed the line of identical cells “HeLa,” after Lacks’s name, and have used it for a variety of medical advancements you’ve likely benefited from. According to the World Health Organization, HeLa cells were used:
- In developing vaccines for polio, HPV, the flu, and Covid-19.
- To study different types of cancer and sexually transmitted diseases, as well as IVF.
It was not until decades later that the Lacks family discovered what had become of her cells.
Thermo Fisher tried several times to have the case dismissed due to a statute of limitation expiration.
The settlement terms are confidential, but Lacks’s family has used the lawsuit to call attention to racism in the American medical system, saying in their complaint, “Too often, the history of medical experimentation in the United States has been the history of medical racism.”
Additional viewing and reading:
- WATCH: How one woman's 'immortal' cells changed the world
- The controversial cells that saved 10 million lives
- Using my family's dark history to teach about vaccines
To read the full BBC story click here.
New Streaming Service Really Has ALL the Stars! (hint: It is from NASA)
NASA Is Launching a Streaming Service
— And It’s Free!

NASA announced last week that it will be launching its own streamer, NASA+, sometime this summer. The family-friendly service will feature the agency’s live coverage of events like spaceship launches, as well as original series showing an inside look at its many ongoing missions. It is completely free (with no ads) and requires no subscription!
“We’re putting space on demand and at your fingertips with NASA’s new streaming platform,” Marc Etkind, an associate administrator for NASA’s Office of Communications, said in a press release. “Transforming our digital presence will help us better tell the stories of how NASA explores the unknown in air and space, inspires through discovery, and innovates for the benefit of humanity.”
The organization didn’t announce an official release date yet, but NASA+ will soon be available on the NASA app as well as on smart TVs and wherever else you prefer to stream. Click here to watch the moving trailer and get a sneak peek at what the service will offer.
Wake Forest Welcomes Medical and PA Students at White Coat Ceremony

Wake Forest University School of Medicine recently proudly welcomed 145 new medical students, from 28 states, during its recent White Coat Ceremony – a cherished tradition that marks the beginning of their journey to become physicians. It also celebrated 86 PA students from the class of 2024.
During the white coat ceremony, L. Ebony Boulware, MD, MPH, dean of Wake Forest University School of Medicine and Chief Science Officer and Vice Chief Academic Officer of Advocate Health, spoke to students about how the white coat symbolizes the values of compassion, trust, inclusion and service – and the highest standards of excellence and professionalism in medicine.
“I wanted a medical school that was focused on service and people, and that also placed an emphasis on how we can use medicine to uplift the voices of others and give dignity to people,” said Lucy Lawrence, a first-year medical student. “I felt like Wake Forest University School of Medicine really prioritized caring for us, even at our Second Look a few months ago, the common denominator through the entire event was talking about how the School is here to support us, care about us as people – not just as doctors – and how they’re going to come along beside us during this process. That was really important to me because this is such a big journey.”
The day was especially meaningful, as the Class of 2027 is the first medical school class that Dean Boulware has had the privilege of welcoming as Dean.
To read more and see a short video click here.
New Bionic Hand Allows User to Control Each Finger With Unprecedented Accuracy
Successful testing of the bionic hand has already been conducted on a patient who lost his arm above the elbow.

(Euronews - Camille Bello and Roselyne Min) -- In a world first, surgeons and engineers have developed a new bionic hand that allows users with arm amputations to effortlessly control each finger as though it was their own body.
The innovation could revolutionize the way prosthetic limbs are designed and used, with scientists hailing it as a “major breakthrough”.
Prosthetic limbs can help people regain some functionality they lost after an amputation, but they can be challenging to control and sometimes unreliable, with only a limited range of motion.
However, for individuals with amputation higher up the arm, such as an above-elbow (transhumeral) amputation, things are more challenging.
In such cases, insufficient muscles remain to generate myoelectric signals to enable control of the lost arm and hand joints, meaning controlling the prosthetic limb in a way that feels natural is simply not possible.
A new era of bionic hands
The key to the new bionic hand - described in a study published in Science Translational Medicine - is a technique called neuromuscular reconstruction.
In this procedure, surgeons rewire the nerves in the residual limb so that they control different muscles. This allows users to generate more complex movements with the bionic hand, such as flexing and extending all five fingers to pick up small objects or type on a keyboard.
“So when the patient tries to do movements with his missing hand, those new muscular structures get activated,” Max Ortiz Catalan, Director of the Center for Bionics and Pain Research in Sweden, Head of Neural Prosthetic Research at the Bionics Institute in Australia, and Professor of Bionics at Chalmers University of Technology in Sweden, told Euronews Next.
“Then we put electrodes inside them so we can extract that information, use an artificial intelligence algorithm to look at those electrical pulses that come from all the different sources and learn what is the patient trying to do. And once it's learned, which happens very quickly, then you can tell the prosthesis what to do,” he added.
Prosthetic limbs are typically attached to the body with a socket that compresses the residual limb making it mechanically unstable and uncomfortable. But the new bionic hand also tackles this problem.
Besides the neuromuscular reconstruction, the new implant also features a titanium implant that is placed within the residual bone connecting it to the body. This provides a more stable and comfortable attachment point for the hand, while also allowing for more natural movement.
"This is a major breakthrough in the field of bionic limbs," said Ortiz Catalan as "it opens up the possibility of creating bionic hands that are as functional as natural hands."
The achievement is based “on over 30 years of gradual development of the concept, in which I am proud to have contributed” said Dr. Rickard Brånemark, a leading expert on osseointegration for limb prostheses, who conducted the implantation of the interface.
‘Commercially available hopefully soon’
The new bionic hand has successfully been tested on a 52-year-old Swedish male patient, Tonney, who lost his arm above the elbow in a workshop. Tonney reportedly learnt to control the implant quickly and easily, as well as perform a variety of tasks with it, including moving the fingers.
In 2020, Tonney and the research team at Chalmers University of Technology participated in Cybathlon, an international athletic competition for people who use assistive technologies.
According to the research team, Tonney has used the prosthetics in his daily life for over three years now and is still using them.
The development of the new bionic hand is a new sign of hope for people with amputations. Close to 60 million people were living with limb amputation due to traumatic causes worldwide in 2017, according to the Institute for Health Metrics and Evaluation.
The device is currently in trials and the research team is actively working with surgeons and professionals in prosthetics around the world to bring this technology to patients.
“This combination [of surgical procedures and the bionic hand] was the first time. But now that we know it's reliable and it can be conducive to better control, people are going to start implementing it,” Ortiz Catalan told Euronews Next.
“The new regulations in Europe have made it a bit more challenging to bring new products to the market. But we hope that we can bring this technology to more people relatively soon,” He added.
The research was led by Professor Max Ortiz Catalan, founding director of the Center for Bionics and Pain Research (CBPR) in Sweden, and the surgery took place at the Sahlgrenska University Hospital, Sweden, where CBPR is located.
For more and to watch a video on the bionic hand click here.
Boost Your Office Moral, One Leaf at a Time!
Research on “micro-nature” demonstrates its positive impact on employee performance and well-being.

Corporations around the globe are realizing that employees need a connection to nature. Google, LL Bean, Facebook, Uber, Nike, and LinkedIn are working to go green in a new way. The understand the benefits of plants in the office. New research on micro-nature, the incorporation of small, affordable natural elements, is showing a positive impact on employees.
A new article in the Harvard Business Review shows that exposure to nature at work boosts productivity, helpfulness, and creativity. It also says there is no evidence of negative side effects.
The research worked with people in the U.S, Canada, China, New Zealand, and Indonesia. The results were consistent, showing that even small doses of nature at work improved the way employees felt. The findings indicate that micro-nature in the workplace can contribute to worker well-being and performance.
To read the article with the full report click here.
For the best plants for your office, according to plant experts, click here.
New Trial Data: Postpartum Depression Pill Shows Positive Effects
Zuranolone is not yet approved by the FDA.

(ABC News Youri Benadjaoud and Sony Salzman) -- A pill for postpartum depression led to meaningful improvements in depression symptoms, according to a late-stage study published Wednesday in The American Journal of Psychiatry.
The pill, zuranolone, is not yet approved, but the Food and Drug Administration is expected to make its approval decision by Aug 5.
In the study, 196 mothers who experienced postpartum depression were split into two groups - half were zuranolone, half given placebo pills for two weeks.
All of the mothers experienced an improvement in their depression symptoms, but those who were taking the real medication experienced significantly better improvements than those who got the placebo pills.
Those improvements were still reported four and six weeks later, according to the study, which was funded by pharmaceutical partners Sage Therapeutics and Biogen.
A small number of patients experienced sleepiness and dizziness.
If approved, zuranolone would be the first pill approved explicitly to treat postpartum depression. An existing treatment called Zulresso was approved in 2019 by the FDA, but that medication is given by intravenous injection.
"It wasn't just that sadness resolved or that crying spells resolved or that sleep improved. But we saw that zuranolone alone was associated with improvement in all of the core symptoms of depression during the course of the study," Dr. Kristina M. Deligiannidis, professor at the Institute of Behavioral Science at the Feinstein Institutes and lead author of the new study, told ABC News.
According to the study authors, the biggest unknown about this pills is the long-term effect — and it's not clear what impact this drug would have on breastfeeding, if any.
Still, experts say one of the biggest barriers to treating postpartum depression isn't medication, but stigma.
"The major issue in postpartum depression is screening and patient acceptance of the diagnosis," said Dr. Jacques Moritz, medical director at Tia women's health.
Meanwhile, existing antidepressant treatments may be helpful for some women, even if those antidepressants are not explicitly approved for post-partum depression.
"Individuals with the first episode of depression may benefit from a standard antidepressant or antidepressant and therapy. Individuals that are on an antidepressant and are not fully responding may depend upon a new option such as this," said Dr. Kimberly A. Yonkers, Departmental Chair and Katz Family Chair, Department of Psychiatry, UMass Chan Medical School/U Mass Memorial Medical Center.
The bottom line, experts said, is that anyone experiencing feelings of depression should seek medical care because treatment and help is available.
If approved, zuranolone would represent an "important incremental addition to currently available treatment options," said Dr. Jessica Shepherd, Chief Medical Officer Verywell Health. But, said Shepherd, "we also need to make sure that the support system is there."
Andrea Sutherland recounted how her third time expecting was filled with constant struggle despite never dealing with depression in her first two pregnancies.
"I wasn't doing any of the regular things that I would do. I wasn't focusing at work, I wasn't taking care of my home, my kids, myself," Sutherland said.
"They wouldn't tell me which [pill] I got. But the minute I got home and took the first pill, I felt like I got the real thing...because I got a sense of relief within the first minutes of me taking it," she added.
Sutherland is among the roughly one in seven women, according to the National Institutes of Health, who develop postpartum depression.
"I would say to a lot of females that might be going through the same thing, that it's real. You are not alone...don't be ashamed," Sutherland said.
"Speak up, try and build that support team around you, express yourself, let others know how you are feeling so they can keep an eye on you. It's normal, it's nothing to be afraid of and there's nothing wrong with getting help," she added.
Dr. Adesola Oje, Dr. Barrington Hwang and Dr. Alexander Garcia contributed to this report.
MAHEC Lifestyle Medicine Symposium Registration Now Open

The Mountain Area Health Education Center is hosting a Lifestyle Medicine Symposium September 30 and registration is now open.
Topics include:
- LM and Brain Health
- Stress Less, Thrive More!
- Health Transformation Through Nutrition
- Cooking demos
- Power of Nature to Reach the Peak of Well-Being
- Panel discussions
The event is Saturday, September 30 at MAHEC, Mary C. Nesbitt Biltmore Campus, Asheville. Check-in begins at 7:30am.
Click here for more information
CMS: US Will Spend $7.2 Trillion on Healthcare in 2031
Healthcare will make up 19.6% of the country’s GDP
(Healthcare Economics - Maia Anderson) -- The numbers are in: The US will spend $7.2 trillion on healthcare by 2031, or 19.6% of gross domestic product (GDP), according to annual national expenditure projections released by the Centers for Medicare and Medicaid Services (CMS) on June 14.
In comparison, the US spent about $4.4 trillion on healthcare, or 17.4% of its GDP, in 2022, CMS estimated.
Rapid growth: The annual US health spend grew 4.3% in 2022, compared to 2.7% in 2021, according to CMS. In 2023, the agency expects spending to grow 5.1%. Between 2022 and 2031, CMS expects spending to grow an average of 5.4% per year.
In 2022, the US economy’s growth actually outpaced national health spending growth. But between 2022 and 2031, the economy is expected to grow 4.6% annually, or 0.8 percentage points slower than healthcare spending.
Historically high coverage: The CMS projections also include data on US insurer enrollment, and the percentage of insured people in the US reached a historic high in 2022 at 92.3%, thanks to an increase in Medicaid and ACA marketplace plans.
But with Covid-19 public health emergency protections expired, the number of Medicaid enrollees is anticipated to fall from 90.4 million in 2022 to 81.1 million in 2025, according to CMS. Even so, the agency expects more than 90% of the US population will remain insured, partially due to a projected increase in private health insurance plans “from enhanced Marketplace subsidies.”
Some more numbers:
- Medicare spending is projected to grow 7.5% per year between 2022 and 2031, faster than other major payers, compared to 5.4% for private payers and 5% for Medicaid.
- Hospital spending is expected to grow faster (5.8% annually) than spending on physician and clinical services (5.3%) and prescription drugs (4.6%) during that same time period.
- Medicare spending is projected to grow 8% in 2023 (compared to 4.8% in 2022), exceeding $1 trillion.
“Recent legislation is anticipated to affect trends in health insurance enrollment and healthcare spending over the next decade,” Sean Keehan, study author and an economist in the Office of the Actuary at CMS, said in a statement. “Altogether, and consistent with its past trend, health spending for the next ten years is expected to grow more rapidly, on average, than the overall economy.”
ECU's Brody School of Medicine 2027 Class is Largest in History

GREENVILLE -- The newest class of students in the Brody School of Medicine at East Carolina University were officially welcomed during the school’s annual White Coat Ceremony on Friday.
The 90 members of the Class of 2027 — all North Carolina residents — were helped into their white coats during the traditional celebration at the Health Sciences Student Center and regaled with messages and well wishes from Brody faculty and leadership.
The Class of 2027, the largest class in Brody history, hails from 36 North Carolina counties and 26 undergraduate institutions; 61% of the class is female, 16% are first-generation college students, and 21% are from minority groups — Black, Native American and Hispanic or Latino — that the Association of American Medical Colleges considers “underrepresented in medicine.” Four are veterans, and four were collegiate student athletes. The class speaks a total of 23 languages.
One New Student Has Ties to NCMS
The Class of 2027 includes a student whose family has already built a legacy in eastern North Carolina health care.
Grant Irons is the great-grandson of regional health care pioneers Fred and Malene Irons and the grandson of Tom Irons, professor emeritus of pediatrics at Brody, medical director of Access East and the N.C. Agromedicine Institute, and interim medical director of physician assistant studies. Tom Irons is a long standing member of NCMS and a past chair of the CPP Advisory Board.

Tom Irons, left, smiles as he stands with his grandson, Thomas Grant Irons III, after receiving his white coat Friday.
Grant Irons was born and raised in Greenville and attended UNC–Chapel Hill, where he earned a degree in biology with minors in chemistry and health and society.
“It is truly humbling to carry on my family’s legacy here in eastern North Carolina,” he said. “I was lucky enough to spend time with my great-grandparents, Drs. Fred and Malene Irons. As I’ve grown up, I continue to learn more about their work and the impact they had on health infrastructure in eastern North Carolina. Their legacy demonstrates the impact of servant leadership. My late grandmother and grandfather, better known as Carol Irons and Dr. Tom Irons, continued this tradition of selfless service. I would not be the man I am today without their guidance as I aim to hold myself to their standard; a life lived with compassion, and humility.”
Tom Irons said he could not be prouder of his grandson’s desire to serve as a physician.
“I’m Grant’s only living grandparent, but I like to believe that today the other three are wearing smiles as big as mine,” he said. “His parents and I could not be more grateful that he has been given this opportunity, or prouder that he has chosen to come to Brody and eastern North Carolina.”
He added that he encourages his grandson and Grant’s classmates to follow their hearts.
“There is nothing easy about this journey you have undertaken, but if you put your heart into it, the rewards will be immeasurable,” he said. “Keep your head down these first two years, lean on each other, ask for help when you need it and never forget that there is no life more noble than a life of service.”
Grant is eager to live out a legacy and mirror his grandfather’s ideals and impact in medicine — but he is also ready to make his own name through the medical school that represents a life goal.
“The opportunity to be a student at Brody means the world to me; it has been a dream of mine for some time,” he said.
Grant Irons sees the bigger picture of eastern North Carolina’s health care landscape and also plans to make a difference through policy.
“Regardless of my career path, I have long been interested in health policy,” he said. “Initially, I was exposed to the importance of institutions through my family. Since I was old enough to understand, I have been fortunate enough to learn about care disparities and their impact on health outcomes.”
As a student at UNC-Chapel Hill, Irons explored the relationship between power, policy and differential health outcomes. He helped lead a chapter of PIH-Engage, a global health organization dedicated to building sustainable health infrastructure. That experience has led him to lead on a variety of fronts.
“As a physician I want to be more than a care provider; I hope to be a leader in my community,” he said. “While significant progress has and continues to be made, care expansion efforts are far from over. There are far too many people still in need.”
To read more about the students and this years class click here.
First Baby Born from Transplanted Uterus Outside of Clinical Trial

The first baby born from a transplanted uterus outside of a clinical trial entered the world this past May, and doctors are now sharing the details. The mother was born without a uterus due to a congenital condition called Mayer-Rokitansky-Küster-Hauser syndrome.
It happened at the University of Alabama at Birmingham.
The transplant, pregnancy, and birth also represent “an incredible milestone” that will open doors for other parents struggling with infertility, the hospital’s Dr. Paige Porrett said in a news release. “This birth is evidence that reassures us that this emerging technology — this innovative therapy — that’s been needed for so long really, truly works,” Porrett said.
The birth signifies that this procedure is now an option when couples are dealing with infertility.
UAB has an extensive article on the process as well as video with the family. Read and watch it all by clicking here.
On The Lighter Side - July 28, 2023, Watermelon, Soccer, and a Haunted Mansion!
Here are some things NCMS employees, members, and the general public are talking about this week!
Cool Off at the Fair Bluff Watermelon Festival!
Since 1986, Fair Bluff has been offering a way to cool off and enjoy one of summer's greatest treats! The 2023 Fair Bluff Watermelon Festival has everything from watermelon eating contests, to watermelon competitions, a street dance, and even a best legs contest just for the guys! Don't miss the parade at 12:30 on Sunday. Schedule of events and entertainment is here.
Ready for a Couples Getaway?

Check out some of the hottest bands in the state in Manteo this Sunday at the Outer Banks is For Lovers Festival. For more information and tickets click here.
US Women's World Cup Continues! USA Still in the Race for a Three-Peat!

One win and one tie and a long way to go! The US women are still looking for a third title. This weekend look for England, China, France, Italy, Switzerland, and others to take the pitch. New up for the US is Tuesday versus Portugal. It airs at 3am on Fox. For a full schedule of games click here.
Hot Concert Summer Continues

Tonight
- Sheryl Crow with Southern Avenue, Beech Mountain Ski Resort, Beech Mountain
- Grizmas, Live Oak Bank Pavilion, Wilmington
- Jodeci, PNC Music Pavilion, Charlotte
Saturday
- Darius Rucker, Kidd Brewer Stadium, Boone
- Walker Hayes, Skyla Credit Union Amphitheater, Charlotte
- Counting Crows, Red Hat Amphitheater, Raleigh
- The Embers, Paramount Theater, Burlington
Sunday
- The Chicks, Greensboro Coliseum, Greensboro
- Darius Rucker, The Cottage at the Crystal Coast, Atlantic Beach
- Middle Kids, Skyla Credit Union Amphitheater, Charlotte
From Barbie's Dreamhouse to The Haunted Mansion!

After a weekend (and week, TBH) of pink, pink, pink from the Barbie movie, it is time for the latest edition of Disney's Haunted Mansion. This time it features LaKeith Stanfield, Tiffany Haddish, Rosario Dawson, and a ton of Hollywood superstars. Find out where and when you can catch starting today by clicking here.
If you have an event you would like added to On The Lighter Side, email Randy Aldridge at [email protected].
State Takes Action To Start Medicaid Expansion October 1
State Takes Action To Start Medicaid Expansion October 1
Launch depends on General Assembly acting by September 1
RALEIGH -- The North Carolina Department of Health and Human Services is moving forward with Medicaid expansion and announcing the anticipated start date of Oct. 1, 2023. This announcement is part of a compromise agreement NCDHHS obtained from the Centers for Medicare & Medicaid Services that will allow the department to move forward with the required public notices for beneficiaries, counties and providers while still awaiting authority from the NC General Assembly.
To launch expansion on Oct. 1, NCDHHS will still require action by the NC General Assembly — either through “de-coupling” expansion from the budget or through an enacted budget — by Sept. 1. The work the department is doing now will reduce the original implementation period, from 90-120 days upon receiving legislative authority, to 30 days so enrollment can begin more quickly.
"We are thankful for leadership and partnership in passing Medicaid Expansion which will save lives, increase access to care and bring billions of dollars to North Carolina," said NCDHHS Secretary Kody H. Kinsley. "Moving forward now sets the department on a path to be able to get health care coverage to thousands of people as soon as possible."
The state’s implementation plan initially was organized around obtaining final authority from the NCGA by June 30. With budget negotiations — to which final authority to move forward with Medicaid expansion has been tied — slipping toward August, the department contemplated strategies to push forward preparations to maximize the benefit for North Carolina.
If NCDHHS does not have authority to move forward by Sept. 1, the earliest fallback date is Dec. 1, 2023, and depending on how late authority is given, it could fall into 2024. As part of the compromise to move forward, NCDHHS is opening the public comment period on the State Plan Amendment for Medicaid Expansion’s Alternative Benefit Plan, a legal document required to be submitted to the federal government. If you would like to submit a public comment visit https://medicaid.ncdhhs.gov/medex-state-plan-amendment-new-medicaid-expansion-20230726/download?attachment.
If given final authority from the NCGA by Sept. 1, Medicaid expansion in North Carolina will increase the eligible population to adults aged 19-64 who have incomes up to 138% of the federal poverty level on Oct. 1. For example, expansion gives health care coverage to single individuals making under about $20,000 a year. Likewise, a family of three earning under about $34,000 combined will now be eligible. Beneficiaries will get care the same way as existing Medicaid beneficiaries and be eligible for the same comprehensive benefits and copays as other non-disabled adults in Medicaid.
NCDHHS appreciates the leadership of the NCGA and Governor Roy Cooper in securing the passage of HB76, which set the state on the path to expand Medicaid and enable access to care for more than 600,000 North Carolinians. Medicaid expansion will be transformative for access to health care in rural areas, for better mental health and for veterans, working adults and their families while bringing billions in federal dollars to the state.
Bronny James is Stable, But What About Other Kids and Cardiac Arrest?

Bronny James is stable after going into cardiac arrest Monday. The 18-year-old son of basketball superstar LeBron James collapsed during practice at the University of Southern California. He is now stable and out of the ICU, according to a statement from the family’s spokesperson.
Bronny is the sixth-ranked point guard in his class and was expected to make his debut for the Trojans during an exhibition tour in Greece and Croatia next month.
Is cardiac arrest in teens and children something to be worried about?
Lindsay May, MD, FAAP, FRCPC and Shaji Menon, MC,MS, FAAP, FACC, FASE wrote in an article for healthychildren.org, that sudden cardiac arrest is rare in young people, but it can happen. According to the Centers for Disease Control and Prevention (CDC), about 2,000 young, seemingly healthy people under age 25 in the United States die each year of sudden cardiac arrest. These deaths leave behind a huge and devastating impact on families and communities. But there are ways to help identify risk factors that can help prevent these tragedies.
What is sudden cardiac arrest?
Sudden Cardiac Arrest (SCA) is a life-threatening emergency that happens from an "abrupt and unexpected loss of heart function leading to loss of consciousness and collapse." SCA can be fatal if not treated within minutes. Survival outside a hospital depends on people nearby calling 911, along with prompt bystander emergency response such as using CPR and automatic defibrillators.
Are there warning signs for sudden cardiac arrest before it happens?
When SCA happens to seemingly healthy young people, there is usually no obvious injury or medical reasons the patient or family knew about. Some young people who suffer SCA may have previously experienced heart-related symptoms, such as shortness of breath, chest pain or fainting, that weren't thought to be anything life-threatening. Others never had any symptoms of heart problems until the SCA event.
When can sudden cardiac death happen to young people?
Sudden cardiac arrest is thought to be a leading cause of death in young athletes, but it also affects young people not involved in organized sports. It can happen during exercise or at rest, or even during sleep. In some cases, young people can die from sudden cardiac arrest days or weeks later from brain damage that happened during the SCA.
What causes sudden cardiac arrest in young people?
Not all causes of sudden cardiac arrest in children and young adults is known, but may include:
- Hypertrophic cardiomyopathy. Usually inherited and often undiagnosed, this is the most common cardiovascular cause of SCA in young people. Muscle cells in the heart's lower chambers, called ventricles, thicken. This can cause abnormal heart rhythm, especially during exercise. Other types of pediatric cardiomyopathy may also play a role.
- Coronary artery abnormalities. Defects in the way the coronary arteries connect to the heart can lead to decreased blood supply to heart muscle during exercise and cause cardiac arrest. Young people with coronary artery abnormalities usually are born with them but may not notice any symptoms until they are older.
- Primary arrhythmias. In people with structurally normal hearts, sudden cardiac arrest can sometimes be caused by undiagnosed genetic conditions that affect the heart's electrical impulses. For example, these include:
- Long QT syndrome. A heart rhythm condition that can cause fast and chaotic heart rhythm.
- Wolff-Parkinson-White syndrome. An extra electrical pathway in the heart creates a detour that can make it pump very fast.
- Arrhythmogenic right ventricular dysplasia (ARVD). With this inherited condition, some of the heart's muscle tissue gets replaced with scar tissue.
- Myocarditis. Usually triggered by an infection, myocarditis means the walls of the heart are inflamed. Most myocarditis cases in children happen when a virus such as an enterovirus gets into the heart. It can also be caused by bacterial, fungal or parasite infections, and allergic reactions to some medications.
- Marfan syndrome. This connective tissue disease can lead to tears in the heart's aortic blood vessel. People born with the condition, who tend to be tall and have long arms, may not realize they have it.
- Commotio Cordis is caused by a blow to the chest directly over the heart at certain points in the heartbeat cycle. It can happen in kids with completely normal hearts. Commotio cordis is more common in sports with projective objects such as baseball, ice hockey and lacrosse.
Are there ways to help prevent sudden cardiac death?
There are steps families and communities can take to lower their risk of sudden cardiac death in young people:
- Regular well-child visits and sports physicals. All children need regular wellness visits with their doctor. These visits are a chance to get a complete physical exam and detailed health history to help identify risk factors that may contribute to SCA. Pre-participation exams also are important, even if a child is not involved in organized sports because gym class and recreational activities can―and should―involve plenty of exercise! A screening form that can be completed anytime by patients and parents with their doctor, to help decide whether a visit with a specialist is needed.
- Know Your Family History. Gather the heart health history of blood relatives (children, siblings, parents, aunts and uncles, nieces and nephews, grandparents and cousins), and share with your pediatrician. This can help guide questions during well-child check-ups and sports physicals.
- Community Life Support Training and Automated External Defibrillators. The AAP supports age-appropriate life-support training, including CPR, for students and school staff. It also encourages having Automated External Defibrillators (AEDs) near athletic and training facilities. In cases of sudden cardiac arrest, AEDs can quickly deliver an electrical shock to return the heart rhythm to normal.
Risk factors for sudden cardiac death that should prompt additional testing:
|
|---|
Should all young people be screened for sudden cardiac arrest risk?
Performing mass screening tests like electrocardiogram (EKG) and echocardiogram (ultrasound of heart) for all young people or in athletes to identify risk factors for sudden death is not currently recommended. The concern is that high false positive test results can cause unnecessary anxiety and additional testing. Also, not all events can be picked up by these tests.
However, recognizing warnings signs and risk factors, accurate medical history-taking, and cardiology and genetic screening for patients at higher risk because of family members with concerning heart conditions or an SCA, can be very effective ways to help reduce the risk of sudden death.
What's the outlook on sudden cardiac arrest statistics in the young?
There is a lack of reliable information about the incidence and cause of sudden cardiac death in young people. However, the CDC recently established a Sudden Death in the Young Case Registry for babies, children and young adults up to age 20. The goal of this registry is to help create a better understanding of how often and why SCA happens in young people—and how to help prevent it.
Remember
Although sudden cardiac death in young people isn't common, even one life lost is too many. Take steps that can help reduce the risk of these tragedies, such as scheduling regular well-child visits, sports physicals and encouraging CPR and AED training in your community.
More information
- Preparing for Sudden Cardiac Arrest in Schools: The Essential Role of AEDs
- Screening All Children and Teens for Sudden Cardiac Death Risk
- Advocating for Life Support Training of Children, Parents, Caregivers, School Personnel, and the Public (AAP Technical Report)
- Pediatric Sudden Cardiac Arrest (AAP Policy Statement)
Meet a Woman Working to End the STEM Gender Gap - One Wikipedia Page at a Time!
Ladies, this one is for you (and little girls too)!

(Nice News - Ally Mauch and Rebekah Brandes) -- Women make up less than a third of the U.S. workforce in science, technology, engineering, and math (STEM) fields, and earn about 15,000 less than their male counterparts, according to the American Association of University Women. There have been various movements to help end this STEM gender gap in recent years, and Jessica Wade is doing her part — mainly through Wikipedia.
The 33-year-old British physicist has written more than 1,700 Wikipedia biographies for little-known female and minority scientists in an effort to highlight their contributions and hopefully encourage more women to join and stay in the field.
“Throughout history, women scientists have been making breakthroughs and world-changing discoveries,” Wade told Nice News. “I wanted to take their stories to an accessible platform that everyone trusts and understands. Wikipedia is the perfect place for that.”
She typed out her first entry about six years ago, The Washington Post reports, after she met and was “massively impressed” by American climatologist Kim Cobb. Since then, she’s spent countless hours searching scientific journals, publications, and other resources for subjects to showcase. And there is no shortage, she said.
“People assume girls don’t choose science because they’re not inspired,” Wade explained in an interview with Today. “Girls are already interested. It’s more about making students aware of the different careers in science and getting parents and teachers on board.”

(courtesy Jessica Wade)
Wade’s Wikipedia additions include mathematician Gladys West, pharmaceutical nanoscience researcher Ijeoma Uchegbu, and June Lindsey, who was instrumental in the discovery of DNA structure in the mid-1900s. And, in a happy twist of fate, Wade has her very own Wikipedia page — written by others.
A research fellow at Imperial College London, she focuses her work on considering new materials to be used within the field of nanotechnology, which includes quantum computers that will help to “transform health care, transportation, and communications, and enhance resilience to cyber threats and climate catastrophes,” she wrote in a Wired article in December.

(courtesy Jessica Wade Instagram)
While that sphere of interest may seem a bit esoteric to a typical adult, Wade also spends her time making science accessible to youngsters: She’s the author of a children’s book entitled Nano: The Spectacular Science of the Very (Very) Small, which was published in September 2021 and features illustrations by Melissa Castrillón.
And her own education in STEM is far from concluded — she gleans plenty of insight about other areas of study just by writing the Wikipedia biographies. “In the process, I actually learn so much science,” she told the Post. “It’s a fun journey.”