You’re invited to join the RSV National Expert Program to learn about a new FDA-approved option for infant RSV prevention August 16th at 7:00 p.m. ET and 7:00 p.m. PT


Join colleagues with questions surrounded newly approved RSV medication for infants.
To register for the online panel discussion click here.
Following the news of FDA-approval of Beyfortus™ (nirsevimab-alip) on July 17th, here are the ACIP recommendations the use of Beyfortus for all infants.
The ACIP recommendation for Beyfortus include1:
- Infants aged <8 months born during or entering their first RSV season are recommended to receive one dose of Beyfortus (50 mg for infants <5 kg and 100 mg for infants ≥5 kg)
- Children aged 8–19 months who are at increased risk of severe RSV disease and entering their second RSV season are recommended to receive one dose of Beyfortus (200 mg)
In addition to the recommendation, the CDC has voted to include Beyfortus in the Vaccines for Children (VFC) program.
- See the ACIP recommendation
- More information is also available in the US Sanofi Press Release
The Beyfortus ACIP recommendation was based on efficacy and safety results from Trial 03 (phase 2b study) and Trial 04 (phase 3 MELODY study). The populations from these clinical trials represented all infants, including healthy full-term infants, premature infants, and infants at higher risk for RSV infection.
INDICATION
Beyfortus is indicated for the prevention of respiratory syncytial virus (RSV) lower respiratory tract disease in:
- Neonates and infants born during or entering their first RSV season.
- Children up to 24 months of age who remain vulnerable to severe RSV disease through their second RSV season.
IMPORTANT SAFETY INFORMATION
Contraindication
BEYFORTUS is contraindicated in infants and children with a history of serious hypersensitivity reactions, including anaphylaxis, to nirsevimab-alip or to any of the excipients.
Please see additional Important Safety Information below.
Please see the full Prescribing Information for complete study design and results.
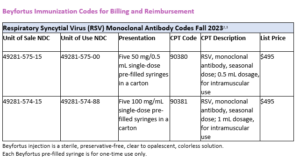
Beyfortus Immunization Codes for Billing and Reimbursement
Click here to find more information on Beyfortus
Please see full Prescribing Information for details.
