NCMS Contingent Meets with Congressman Don Davis

Capitol Chronicle
September 2023
Advocacy Engagement with Congressman Don Davis
Congress’ recent recess afforded the opportunity of scheduling in-district meetings with those who represent North Carolina in the US House of Representatives. One such meeting was with first-term member representing North Carolina’s 1st Congressional District, Rep. Don Davis. Rep. Davis was elected in 2022, having served in the N.C. Senate.
Meeting participants included: Lacy Hobgood, MD / Greenville, NC; Jennifer Parker-Cote, MD / Greenville, NC; Martha Chesnutt, MD / Rocky Mount, NC; Robert Frere, MD / Greenville, NC; and W. Alan Skipper, CAE / NCMS Vice President, External Affairs.
Issues discussed included:
- Medicare Physician Payment Reform – inflationary adjustment
- Prior Authorization relief for patients and physicians
- Step Therapy’s impact on patient care and outcomes
- Workforce needs for North Carolina

Pictured: Hobgood, Frere, Davis, Parker-Cote, Chesnutt, Skipper
_________________________________________________________________________________
Do you know your state and federal legislators? More importantly, do your legislators know you?
The NCMS can help you connect with policy makers as a constituent and advocate!
_________________________________________________________________________________
Support our political advocacy efforts
Be a Key Contact for your legislators

CMS Seeks Public Input on Coverage of Over-the-Counter Preventive Services and more

The Departments of Health and Human Services, Labor, and the Treasury (the Departments) are seeking public input on how best to ensure coverage and access to over-the-counter (OTC) preventive services, including the benefits of requiring most health insurance plans to cover these services at no cost and without a prescription by a health care provider. This new Request for Information (RFI) solicits comment on access to a range of OTC items recommended by experts for preventive care that can be purchased without a prescription, including contraceptives, tobacco smoking cessation products, folic acid during pregnancy, and breastfeeding supplies.
Under the Affordable Care Act, most plans and issuers must cover certain recommended preventive items and services at no cost. Several of these recommended preventive items and services are currently available to consumers OTC without a prescription but are not required to be covered without cost sharing unless prescribed by a health care provider. The goal of the RFI is to understand the potential challenges and benefits for various interested parties, including consumers, plans, issuers, pharmacies, and health care providers, to provide coverage at no cost for recommended OTC preventive products without requiring a prescription. The Departments are committed to ensuring that everyone is able to access affordable and critical preventive items and services, including OTC preventive products.
“All Americans deserve access to quality health care. We know that making preventive care available over the counter can improve access – but there may still be cost barriers. That’s why we are working with the Department of Labor and Department of the Treasury to better understand how a policy change that could further increase access to affordable, preventive care might affect consumers, pharmacies, and health insurance providers,” said HHS Secretary Xavier Becerra. “I hope everyone who might be impacted will submit their comments and help us advance equity in access to high-quality preventive care like contraception and tobacco cessation.”
“The Centers for Medicare & Medicaid Services (CMS) remains steadfast in its commitment to advancing health equity. Easing financial barriers to preventive health care items, without a prescription, is one way to help achieve this goal,” said CMS Administrator Chiquita Brooks-LaSure. “Public input on this change to current policy is vital, and we look forward to hearing from consumers, plans, issuers, and providers about its potential impact.”
The Biden-Harris Administration is committed to ensuring access to health care and addressing health equity across its programs. Having access to affordable, evidence-based preventive items and services is key to promoting the health and well-being of all people. Issuing this RFI is consistent with President Biden’s executive orders on Strengthening Access to Affordable, High-Quality Contraception and Family Planning Services (June 23, 2023), Strengthening Medicaid and the Affordable Care Act (January 28, 2021), and Continuing To Strengthen Americans’ Access to Affordable, Quality Health Coverage (April 25, 2022).
The Departments hope that the comments received in response to the RFI will expand their understanding of the potential health equity effects of requiring coverage for OTC preventive products, without cost sharing and without a prescription by a health care provider, in addressing systemic racism and historic inequity for women and LGBTQIA communities. There will be a 60-day comment period. To be assured consideration, comments must be received at one of the addresses provided in the Request for Information 60 days after date of publication in the Federal Register.
For more information on how to submit comments or to review the entire rule, visit the Federal Register https://www.federalregister.gov/public-inspection/current.
The RFI submitted to the OFR is available now at: https://www.cms.gov/cciio/resources/regulations-and-guidance/downloads/cms-9891-nc.pdf
NCMS LEAD MedTalk Speaker Showcase: Carla Holder, MD
NCMS Welcomes 2023 Leadership College Scholar
Carla Holder, MD
Join us at the 2023 LEAD Conference and Golden Stethoscope Awards Gala Next Month!
Hear Dr. Holder's MedTalk: "Knowledge is Power: Addressing Social Determinants of Health by Increasing Access to Newer, Anti-Diabetic Medications in African Americans with Type 2 Diabetes Mellitus"
To learn more about leadership training opportunities at NCMS click here.
To register for the 2023 LEAD Conference and Golden Stethoscope Awards Gala or to become a sponsor click here.
Registration Now Open for NCTracks October 2023 Provider Training Courses
Registration is now open for NCTracks October 2023 Provider Training Courses
 The first training, New Office Administrator, will be held on Tuesday, October 10 from 9:30 a.m. – 11:30 a.m. This course shows authorized users the process for changing the current Office Administrator (OA) to a new Office Administrator for an Individual Provider or Organization with a National Provider Identification (NPI) number or Atypical Provider. At the completion of training, authorized users will be able to:
The first training, New Office Administrator, will be held on Tuesday, October 10 from 9:30 a.m. – 11:30 a.m. This course shows authorized users the process for changing the current Office Administrator (OA) to a new Office Administrator for an Individual Provider or Organization with a National Provider Identification (NPI) number or Atypical Provider. At the completion of training, authorized users will be able to:
- Update the Office Administrator for an Individual Provider Organization
- Upgrade existing Users to Managing Relationships
Additional Courses offered this month include:
- Prior Authorization Institutional
- Prior Approval Medical
- Changes to Prior Approval Orthodontic Models: Orthodontic (NEW COURSE)
- Submitting Professional Claims
- Submitting a Time Limit Override
Please see the document linked below for more information on course schedule and access to zoom links:
October 2023 Provider Training Schedule
DON'T BE ALARMED! Nationwide Emergency Phone Alert Test Set for Wednesday, October 4.
Test Messages Will be Sent to All TVs, Radios and Cell Phones on Wednesday, October 4.

WASHINGTON -- FEMA, in coordination with the Federal Communications Commission (FCC), will conduct a nationwide test of the Emergency Alert System (EAS) and Wireless Emergency Alerts (WEA) this fall.
The national test will consist of two portions, testing WEA and EAS capabilities. Both tests are scheduled to begin at approximately 2:20 p.m. ET on Wednesday, Oct. 4.
The WEA portion of the test will be directed to all consumer cell phones. This will be the third nationwide test, but the second test to all cellular devices. The test message will display in either English or in Spanish, depending on the language settings of the wireless handset.
The EAS portion of the test will be sent to radios and televisions. This will be the seventh nationwide EAS test.
FEMA and the FCC are coordinating with EAS participants, wireless providers, emergency managers and other stakeholders in preparation for this national test to minimize confusion and to maximize the public safety value of the test.
The purpose of the Oct. 4 test is to ensure that the systems continue to be effective means of warning the public about emergencies, particularly those on the national level. In case the Oct. 4 test is postponed due to widespread severe weather or other significant events, the back-up testing date is Oct. 11.
The WEA portion of the test will be initiated using FEMA’s Integrated Public Alert and Warning System (IPAWS), a centralized internet-based system administered by FEMA that enables authorities to send authenticated emergency messages to the public through multiple communications networks. The WEA test will be administered via a code sent to cell phones.
This year the EAS message will be disseminated as a Common Alerting Protocol (CAP) message via the Integrated Public Alert and Warning System-Open Platform for Emergency Networks (IPAWS-OPEN).
All wireless phones should receive the message only once. The following can be expected from the nationwide WEA test:
- Beginning at approximately 2:20 p.m. ET, cell towers will broadcast the test for approximately 30 minutes. During this time, WEA-compatible wireless phones that are switched on, within range of an active cell tower, and whose wireless provider participates in WEA, should be capable of receiving the test message.
- For consumers, the message that appears on their phones will read: “THIS IS A TEST of the National Wireless Emergency Alert System. No action is needed.”
- Phones with the main menu set to Spanish will display: “ESTA ES UNA PRUEBA del Sistema Nacional de Alerta de Emergencia. No se necesita acción.”
WEA alerts are created and sent by authorized federal, state, local, tribal and territorial government agencies through IPAWS to participating wireless providers, which deliver the alerts to compatible handsets in geo-targeted areas. To help ensure that these alerts are accessible to the entire public, including people with disabilities, the alerts are accompanied by a unique tone and vibration.
Important information about the EAS test:
- The EAS portion of the test is scheduled to last approximately one minute and will be conducted with the participation of radio and television broadcasters, cable systems, satellite radio and television providers and wireline video providers.
- The test message will be similar to the regular monthly EAS test messages with which the public is familiar. It will state: “This is a nationwide test of the Emergency Alert System, issued by the Federal Emergency Management Agency, covering the United States from 14:20 to 14:50 hours ET. This is only a test. No action is required by the public. [source]
NCTracks: Copays No Longer Required for Adult Vaccines and Vaccine Administration
As of Oct. 1, 2023, copays are no longer required for adult vaccines and vaccine administration.
On June 27, 2023, the Centers for Medicare & Medicaid Services (CMS) released guidance on mandatory coverage requirements for adult vaccines.
As part of this guidance based on statutory amendments made by section 11405 of the Inflation Reduction Act (IRA), the Advisory Committee on Immunization Practices (ACIP) recommended adult vaccines and their administration are to be provided without cost sharing.
Questions? Contact NCTracks Call Center: 800-688-6696
Mental Health Resource Guide for LGBTQIA2S+ Youth

The federally supported Suicide Prevention Resource Center is producing a series of guides to support the mental well-being of Lesbian, Gay, Bisexual, Transgender, Queer, Intersex, Asexual, and Two-Spirit (LGBTQIA2S+) youth.
The first in the series, Resource Guide for Professionals, Families, and Communities, focuses on resources for professionals, families, and communities.
The series includes a resource guide and four companion focus guides designed for specific populations. Developed by SPRC in partnership with NORC at the University of Chicago, the guides center the input of LGBTQIA2S+ individuals and those who have experienced suicidal thoughts and behaviors.
Upcoming in the series:
- Focus Guide for Parents, Families, and Communities
- Focus Guide for Health and Behavioral Health Professionals
- Focus Guide for School Professionals
- Focus Guide for State Agencies
Annual Miles for Mammos 5k Returns October 28

On October 28, 2023, the Onslow Memorial Hospital Foundation, in partnership with Onslow Memorial Hospital Women’s Imaging, will host the annual Miles for Mammos 5k at the Jacksonville Commons in support of Julie’s Pink Warrior Project.
Julie’s Pink Warrior Project provides free screening mammograms to the underinsured and uninsured in Onslow County. Since its inception in 2018, Onslow Memorial Hospital Foundation has provided over 395 mammograms.
“This race is gearing up to be the biggest yet,” said Jennifer Provost, Director of Onslow Radiation Oncology. “We have had amazing support in sponsorships and have exceeded our fundraising goal for the first time in years; we have over 90 people registered, and we expect that number to grow in the coming weeks.”
The Onslow Memorial Hospital Foundation Project is named after Julie Zerkle, a breast cancer warrior who lost her battle with cancer at 38 years old. The Foundation along with Onslow Memorial Hospital employees created this fund because they identified access to screening mammograms as a barrier for the under and uninsured. Julie’s Pink Warrior Project saw its first patient on July 3, 2018, and has been helping women in Onslow County ever since.
In 2020, Julie’s Pink Warrior Project began partnering with the Onslow County Health Department to bring the Breast Cervical Cancer Control Program (BCCCP) back to Onslow County. The BCCCP program is a community-based partnership created to help provide uninsured or underinsured individuals who face high out-of-pocket costs due to insurance deductibles full access to breast and cervical cancer screening.
To support Julie’s Pink Warrior Project and register for Miles for Mammos 5k on Saturday, October 28, 2023, at the Jacksonville Commons, visit: https://runsignup.com/Race/Events/NC/Jacksonville/MilesforMammos5kand1mileFunRun.
Spots on race day may be limited. If you are interested in setting up a vendor booth for this event, please contact: [email protected]
HRSA Launches New Website in Espanol
A new HRSA website includes a centralized repository of Spanish-translated, HRSA-produced resources and webinars.

BPHC-related resources include the BPHC compliance manual, a video on Scope of Project, and a widget for the Spanish-language “Find a Health Center” tool. Additional materials and webinars will be added to the website over the coming months.
Este nuevo sitio web incluye un depósito centralizado de recursos y seminarios web traducidos al español y producidos por HRSA. Los recursos relacionados con BPHC incluyen el manual de cumplimiento, un video sobre el alcance del proyecto y un widget para la herramienta “Encontrar un centro de salud”. Se agregarán materiales y seminarios web adicionales al sitio web en los próximos meses.
Visit the website here.
Happy Birthday to These NCMS Members Celebrating This Month!
Grab your party hats and noise makers and let’s celebrate!
John L. Abernethy, Jr., MD, PhD
Robert M. Adams, IV, MD
Feyisayo A. Adeyina, MD
Kamal S. Ajam, MD
Jamie H. R. Aldridge, PA
Shannon F. Alejandro, MD
Zulfiqar Ali, MD
Gilbert R. Alligood, Jr., MD
Peter D. Almirall, MD
Charles M. Almond, MD
George J. Alter, MD
Theresa C. Amerson, MD
William K. Andersen, MD
J. Robert Anderson, MD
Pooja J. Apte, MD
Sasan S. Araghi, MD
Gerald M. Aronoff, MD
Kandis D. Atkins, PA-C
Michael J. Azrak, MD
Claxton A. Baer, MD, PhD
D. B. Baird, MD
Matthew G. Baker, PA-C
Scott W. Baker, MD
Marquiez D. Ballard, PA-C
Robert M. Barr, MD
David W. Barry, MD
Derrick A. Bass, PA-C
Michael P. Battaglino, MD
Jennifer C. Baxley, PA-C
Carmen J. V. Beamon, MD, MPH
Stacey B. Bean, MD
Ann Y. Becker, MD
Ross J. Bellavia, MD
W. Tyson Bennett, MD, FACC, FACP
Jill L. Benson, MD
Thomas W. Benton, MD
Jerry C. Bernstein, MD, FAAP
David R. Bierman, MD
Gary L. Biesecker, MD, FACS
Sukanto Biswas, MD
Billy G. Black, MD
Charles W. Blount, Jr., MD
L. A. Boardman, MD, FACOG
James D. Bobbitt, MD
Casey E. Bohl, MD
R. Randal Bollinger, MD, PhD
Jeremy J. Bonkowske, MD
Alexandra H. Boster, DO
Carol B. Bounajim, MD
Josie B. Bowen, MD, FACEP
W. Scott Bowie, MD
Richard F. Bowling, MD
James F. Bowman, MD
Dale W. Boyd, Jr., MD
Michael Bradshaw, MD
James D. Branch, MD
Teresa S. Bratton, MD
Jacob R. Brayboy, MD
Mark E. Brenner, MD
Bradley C. Brenton, MD
Thomas D. Bresley, MD
Thomas E. Brewington, Jr., MD, JD
Don C. Bright, MD
Mary D. Broga, MD, FAAP
Jennifer A. Brooks, PA-C
Chamaine R. Brooks-Locklear, MD
Thomas M. Brosnan, MD
Delores E. Brown, MD
Elizabeth D. Brown, MD
Thomas L. Brown, MD
J. Dale Browne, MD
John H. Buck, MD
Manuel E. Bulauitan, MD
Julia A. Bulkeley, MD
Eithne T. Burke, MD
Timothy A. Burke, MD
Walter Woodrow Burns, Jr., MD
Craig M. Burnworth, MD
Edwin R. Cadet, MD
Molly S. Calabria, PA
A. Barry Campbell, MD
Stephen J. Capps, MD
C. Bradley Carlson, MD
Richard K. Carmona, MD
William C. Carr, MD
Marc R. Carruth, MD
Lawrence S. Carter, Jr., MD
Alden K. Casati, PA-C
Thomas E. Castelloe, MD
Stephanie A. Cernuto, PA-C
Dana L. Chambers, MD
J. Kenneth Chance, MD
Kerry E. Chandler, MD
Cynamon K. Chawla, MD
Firas Chazli, MD
C. Frank Chen, MD
Qing Chen, MD
Nirav Chiniwalla, MD
Paul S. Chipley, MD
Matthew J. Chovaz, MD
Tara L. Chronister, MD
Badie T. Clark, III, MD
Olivia M. Clelland, PA-C
Kelly M. Clifford, MD
W. Gerald Cochran, MD
Max W. Cohen, MD
Samuel D. Cohen, MD
Devon J. Cole, MD
Teddy G. Combs, PA-C
Patrick M. Connor, MD
Anna J. Conterato, MD
Brendan M. Corcoran, MD
Scott L. Cornella, MD
Thomas P. Cornwall, MD
Daniel T. Cotter, MD
Benjamin L. Coulter, MD
Mary L. Courrege, MD
Christopher D. Covington, DO
Emily J. Cox, DO
Kathryn H. Cox, MD
John T. Crawford, MD
Harry D. Crews, MD
Laddie M. Crisp, Jr., MD
Luis J. Cuervo, MD
Julie A. Czech, MD
Kunal S. Dalal, MD
Christopher T. Daley, MD
Nicole M. D'Andrea, MD, MPH, FACOG
Robert D'Angelo, MD
Walter E. Daniel, MD
David M. Dare, MD
Leroy S. Darkes, MD
Deborah H. Davis, MD
Keith E. Davis, MD, FACC, FACP
Michael E. Davis, MD
Samuel P. Davis, III, MD
Katyucia de Macedo Rodrigues, MD
Thomas W. deBeck, MD
R. Prasad DeGala, MD
Larry C. Dekle, MD
Megan A. DeMariano, MD
Nilay V. Desai, MD, FACE
Ronald W. Digby, MD
John H. Dilworth, MD
Dobrinka V. Dimitrova Koutleva, MD
Meredith G. Disharoon, DO
S. Shripad Dongre, MD
S. Trevor Downs, PA-C
John E. Drew, MD
Logan S. D'Souza, MD
Todd H. Duncan, MD
Andrea M. DuPont, MD, FACEP
Sira M. Duson, MD
Suzanne E. Dvergsten, MD
Robert R. Earnest, MD, FAAP
John Stewart G. Edmunds, MD
Bradley S. Ellison, MD
Scott C. Elston, MD
Carrie A. Enright, PA-C
Celia B. Entwistle, MD, FACEP
C. Allan Eure, MD
J. Holt Evans, MD
James C. Fahl, MD
Amanda L. Faulkner, MD
David E. K. Feldman, MD
Nathan P. Fergus, MD
Ashley M. Ferguson, MD
Michael O. Ferguson, MD
Lynne C. Fiscus, MD, MPH
Jessica M. Fisher, MD
Thomas F. Flaherty, MD, MPH
T. Sledge Floyd, Jr., MD
Philip B. Fontenot, MD
Clayton J. Foret, MD
Robert M. Foster, MD
Tanaya P. Foster, PA-C
Barry I. Freedman, MD
Preston H. Gada, MD
Louis A. Gagliano, MD
Paul G. Galentine, III, MD
Jeffrey Michael B. Galvin, MD
W. Ray Gammon, MD
Lisa M. Gangarosa, MD
Ronald L. Garber, MD
Gilbert J. Garcia, Jr., MD
Brian P. Garvin, MD
Christopher P. Garwacki, MD
R. Glenn Gaston, MD
Paige L. Gausmann, MD
Laura C. Gay, MD
Robert M. Gay, MD
Alfred E. Geissele, MD
Richard W. Geldmeier, MD
Elizabeth J. Geller, MD
Ryan D. Gentry, MD
Gregory R. Gibbons, MD
David E. Gibson, MD
Thomas V. Giguiere, MD
M.G.F. Gilliland, MD
Carrie G. Gill-Murdoch, MD
Kathryn M. Godly, PA-C
Keely B. Godwin, MD
Meredith O. Godwin, MD
Paul A. N. Gordon, MD
Kathryn R. Gordon-Escobar, PA-C
Manish Goyal, MD
Soledad C. Griffin, MD
James G. Groce, MD
Stephanie A. Grotzke, MD
Jessica M. Gruenberg, MD
Carmelo Gullotto, MD
Anna B. Gulyn, MD
Sandeepkumar J. Gupta, MD
Regina E. Gurley, PA
Bhavna K. Gvalani, PA-C
William D. Hage, MD
Warner L. Hall, Jr., MD
Frank T. Hannah, MD
Kathleen W. Harknett, MD
David K. Harper, MD
Wayne W. Harper, MD
Charles W. Harris, MD
James M. Harris, MD
Jason R. Harris, MD
Anastasia Hastie, PA-C
Chester C. Haworth, Jr., MD
William C. Hayes, Jr., MD
Hubert B. Haywood, III, MD, FACP, FIDSA
Michael N. Heacock, MD
Paul J. Healy, MD
James R. Hedgepeth, MD, FAAP
Edward A. Hedrick, MPAS, PA-C
Jeanette H. Hemp, MD
Shane D. Hemphill, MD
Thomas F. Henley, MD
Georgia A. Hennessy, MD
W. D. Henrichs, MD
John M. Herion, MD
Darrell E. Hester, MD
David A. Hester, MD
Frederick A. Hewett, II, MD, FAAP
Caitlin M. Higgins, MD
Michael D. Hightower, MD
Andrew U. Hines, MD
Micaela M. Hofer, PA-C
Byron J. Hoffman, Jr., MD, MACP
Colleen M. Holden, PA-C
Jennifer L. Holmes, MD
Dimitrios P. Hondros, MD
John D. Howell, Jr., PA
Russell M. Howerton, MD
Jeffrey A. Huang, MD
Syed H. Hussaini, MD
Russell A. Incatasciato, DO
William F. Ingram, III, MD
Mohammad O. Iqbal, MD
Sangeetha Isaac, MD
Arin L. Isenstein, MD
Harold N. Jacklin, MD
William A. Jarrett, MD, FACS
Jana G. Johnson, MD
Kenneth L. Johnson, MD
Robin T. Johnson, PA
Curtis B. Johnsrude, MD
Colin D. Jones, MD
Drew A. Jones, MD
Gilbert K. Jones, MD
Jeffrey D. Jones, MD
John R. Jones, MD
Venkata R. Jonnalagadda, MD, FAPA
Ryan M. Jordan, DO
Damilola O. Joseph, MD
Donald G. Joyce, MD
Carmin M. Kalorin, MD
Mukesh N. Kamdar, MD
Robert E. Kanich, MD
William S. Kaufman, MD
Catherine M. Kelly, MD
Todd L. Kelly, MD
Ashton S. Kennedy, PA-C
Brian H. Keogh, Jr., MD
Harold B. Kernodle, Jr., MD
Saleen Khan, MD
Larry S. Kilby, MD
Karen E. Kimel-Scott, MD
Mary J. Kirby, MD
Sidney E. Kirkley, MD
Joseph W. Kittinger, III, MD
Michelle E. Klawiter-Benton, MD
George Klein, MD
Aaron D. Kline, MD
Laura M. Kline, MD
Michael W. Klinkner, MD
Thomas M. Knutson, MD
L. A. Koman, MD
William L. Kozel, MD
Ernest N. Kraybill, MD
Ted R. Kunstling, MD, FCCP
Nicholas D. Kurtzman, MD
Richard W. Kurzmann, MD
Jeffrey T. Kuwahara, MD
Anthony J. Kwon, MD
Kimberly Kylstra, MD
Jeffrey A. Lamphere, PA-C
Charley W. Lane, PA-C
John G. Langhenry, IV, MD
Michael R. Lawless, MD
John R. Leaton, DO
Tae J. Lee, MD
Baxter C. J. Leonard, MD, FAAFP
William F. Lestini, MD
Stuart J. Levin, MD
Peter M. Levitin, MD
Caroline M. Lewis, DO
Timothy E. Lietz, MD, FACEP
David M. Lingle, MD
Mark D. Lins, MD
James C. Little, Jr., MD
Gandhari Loomis, DO
Robert R. Lopez, MD
James M. Love, MD
Kent V. Lucas, MD
Stephen D. Lucey, MD
John B. Lykes, MD
Joshua C. Macomber, MD, FACC
Kenneth R. Madsen, MD
Robert P. Majors, Jr., MD
Heidi C. M. Mangelsdorf, MD
Jayadev Manikkam Umakanthan, MD
James T. Mann, III, MD
James R. Manning, III, MD
Gregory A. Mantooth, MD, FACS
Susan L. Marra, PA-C
Sheila F. Marshall, DO
Charles T. Marston, Jr., MD
Richard W. Martin, MD
John L. Masonis, MD
Clinton E. Massey, MD
Tushar Mathur, MD
David C. Matthews, MD
John Matzko, MD
Richard J. Max, MD
James A. McAlister, Jr., MD
Greig V. McAvoy, MD
Patrick S. McElgunn, MD, MBA
James S. McFadden, MD
Douglas S. McFarlane, MD
Jacquelyn A. McGill, MD
Murphy F. McGirt, Jr., MD
Franklin R. McGuire, MD
Patricia L. McHale, MD
John W. McKay, MD
Netasha S. McLawhorn, MD
William W. McLendon, MD
Michele H. McMillan, MD, FAAP
S. Dean McPhail, MD
Michael J. McWilliams, MD
Joseph W. Melamed, MD
Morton Meltzer, MD
Rukmini Menon, MD
Mindy L. N. Merritt, MD
Terry M. Messer, MD
Pradeep Mettu, MD
Kai Miao, MD
Kenneth J. Michau, II, MD
Adam R. Militana, MD
Philip R. Miller, MD
Chadwick M. Mills, MD
Duy An T. Minior, MD
Leanne F. Minnick, PA-C
Mohit Mody, MD
Anita M. Molby, DO
Richard E. Moon, MD
Robert B. Moore, MD
B. Dawn Moose, MD
Greta S. Morcos, PA-C
Lauren W. Morris, PA-C
Leon M. Morrison, MD
Ashley M. Mortenson, MD
Robert G. Moser, MD
Michael P. Moulton, MD
Kristen M. Mundy, PA-C
Martin J. Murphy, MD
Jason A. Mutch, MD
Francis A. Neelon, MD
Marc H. Nesi, MD
Dale A. Newton, MD
Gunjan Nigam, MD
James M. North, MD
Sean P. O'Brien, MD
Imelda N. Odibo, MD, FACOG
Kenneth G. Olsen, MD
Michael A. Olympio, MD
Brian M. Opalacz, DO
Suzette R. Oxendine, PA-C, MMS
Aurora K. Pajeau, MD, MPH
Gaurang M. Palikh, MD, FAAN
Jeffress G. Palmer, MD
Robert M. Palmer, MD
Laurie S. Panzer, PA-C
Rohan M. Parekh, PA-C
Amrita Parikh-DeSai, MD
Bill J. Parker, MD
David W. Parker, II, DDS, MD
R. Lamar Parker, Jr., MD
Jerome P. Parnell, II, MD
Marshal R. Parsons, MD
Mohammed Afraz Pasha, MD
Jacek J. Paszkowiak, MD
Darshan B. Patel, MD
Praneil Patel, MD
Ravenne A. Patel, MD
Sachin B. Patel, MD
Shital M. Patel, MD
Susanj S. Patel, MD
Jerry E. Patterson, MD
C. Charese Pelham, MD
Prabhakar D. Pendse, MD
Philip S. Perdue, Jr., MD
Lenin J. Peters, MD
John L. Peterson, MD
Louis W. Pettygrove, PA-C
Edward M. Pickens, MD
Eduardo A. Piqueras, MD
Eric W. Pittman, MD
Jeffrey Pokorny, MD
Matej Polomsky, MD
Henry A. Pool, MD
William L. Porfilio, MD
Charles R. Port, DO
Thomas L. Presson, Jr., MD
Ronald A. Preston, MD
Douglas C. Privette, MD
Ronald J. Prucha, Jr., MD
Richard W. Puschinsky, MD
Meghan K. Pyle, MD
Katharine A. Pyron, MD
Shawn P. Quillin, MD
Aamer A. Qureshi, MD
Abu-Ahmed Z. Rahman, MD, FACP
Michael G. Rallis, MD
E. Allison Ramsey, MD
Tom S. Rand, MD
Stewart F. Rasmussen, MD
Janakiram Ravulapati, MD
Patrick M. Reames, MD
Michael A. Reardon, MD
Marshall S. Redding, MD
Monica B. Reddy, MD
Kara A. Regan, MD
Robert L. Reid, MD
David A. Rendleman, III, MD
James M. Rhyne, MD
John C. Rickabaugh, MD
Miriam E. Ridley, MD
Waldemar L. Riefkohl, MD
Craig A. Rineer, MD
Eric C. Ringwalt, MD
George E. Rinker, MD
Janet L. Rippel, PA-C
April M. Risinger, PA-C
John G. Roach, III, MD
Karen Y. Robinson, MD, FAAP
Laurian S. Roediger, MD
Ryan C. Romano, DO
Sara L. Rooker, MD
Brandon S. Rorie, PA-C
J. Carson Rounds, MD
J. Lawrence Rouse, III, MD
Brandon P. Roy, MD
Michael R. Ruffolo, MD
Jeffrey W. Runge, MD, FACEP
Timothy E. Ryan, MD
Samy R. Saad, MD
Frank Sabiston, Jr., MD
George H. Salama, MD
Richard Sanchez, MD
Jos R. Santz, I, MD
Thomas R. Saullo, MD
Charles D. Scheil, MD
John L. Scheitler, MD
Irvin G. Scherer, MD
Neil E. Scheurich, MD
Jessica N. Schloesser, DO
Jeffrey S. Schmidt, MD
Andrew M. Schulman, MD
Lindsey K. Seaver, PA-C
Paul B. Segebarth, MD
Monica A. Selak, MD
Andrew T. Selfe, PA-C
Scott D. Sexton, MD
Charnette H. Shade, MD
Disha N. Shah, DO
Rickin A. Shah, MD
Nasfat Shehadeh, MD
Pheston G. Shelton, IV, MD
John D. Shepherd, MD
James D. Shumate, DO
Sufia Siddique, MD
Adeel M. Siddiqui, MD
C. Van Sikes, III, MD
William J. Simons, MD
Jason F. Simpson, MD
Ronald W. Singer, MD
Puneet P. Singh, MD
Jay A. Singleton, DO
Anthony F. Skalak, MD
Scott C. Sledge, MD
Anne B. Smith, MD
Ellison L. Smith, MD
Hannah C. Smith, PA-C
Karen L. Smith, MD, FAAFP
Whitman E. Smith, Jr., MD
Douglas J. Snyder, MD
Clinton R. Soriano, MD
Mark O. Speight, MD
Joel F. Spragins, MD
Robert K. Stack, MD
John A. Stahl, MD
J. Andrea Staneata, MD
John H. Stanley, Jr., MD
Rodney J. Stanley, MD
Charles E. Stoddard, III, MD
Bradley J. Stoneking, MD
Christian J. Streck, MD
Leah R. Strickland, MD
Timothy D. Stuart, DO
Helena G. Summers, MD
Brian A. Sumner, MD
Sever C. Surdulescu, MD
Gregory M. Swank, MD, FACS
Brooks W. Taber, MD
David E. Tart, MD
David T. Tayloe, III, MD, FAAP
Blucher E. Taylor, MD
Richard L. Taylor, MD
Trevor M. Taylor, MD
S. Eldora Haworth Terrell, MD
T. Eugene Terrell, MD
Andrew M. Terzian, MD
Debra J. Tetreault, MD
Zsuzsanna P. Therien, MD
Dimitri M. Thomas, MD
Henry C. Thomason, Jr., MD
Allie Y. Thompson, PA-C
Lisa W. H. Thompson, MD
David C. Thornton, MD
Lindsey N. Thornton, DO
Thomas G. Thurston, III, MD
George M. Tosky, MD
James L. Toussaint, MD
Charles E. Trado, MD
Henry W. Traylor, Jr., MD
James M. Tsahakis, MD
Alex Y. C. Tse, MD
Matthew K. Tsuei, MD, FACS
Mary Catherine Turner, MD, FACP, FAAP
Pamela A. Turpin, PA-C
Marili Uno Witt, MD
Daniel S. Uri, MD
Michael J. Utecht, MD, FACEP
Henry W. Van Gils, PA-C
Fred H. Van Nynatten, MD
Mark D. Van Poppel, MD
John H. Vance, MD
Shona S. P. Varghese, MD
Bradley K. Vaughn, MD
Reid C. Vegeler, MD
Kinga M. Vereczkey, MD
John D. Vrnak, PA-C
Phuong-Mai J. T. Vu, MD
Scott D. Wait, MD
Phillip J. Walker, MD
Thomas L. Warren, MD
Ronald P. Waterer, MD
Jerry L. Watson, MD
Seth F. Weaver, II, MD
Dakota R. Webster, PA
Rebecca Y. Weinshilboum, DO
Michael S. Weizman, MD
Briana H. Welch-Denton, PA
Brittany L. West, PA-C
Mark J. Whalen, MD
Anthony H. Wheeler, MD
James H. Whicker, MD
Thomas H. White, MD
Russell R. Wilford, Jr., MD
Carey C. Williams, MD
Daniel M. Williams, MD
Emily C. Williamson, MD
Warren L. Williamson, MD
Larry F. Willis, MD
Paul A. Willman, DO
Brett L. Wilson, MD
Lisa K. Wilson, MD
Robert B. Wilson, II, MD
James V. Winkley, MD
Christopher A. Winter, MD
Benjamin M. Wooster, MD
S. Lamont Wooten, MD
Matthew Wrench, DO
David O. Wright, MD
Makram A. Yassa, MD
Pavan K. Yerramsetty, MD
Ming Yin, MD, PhD
Andrew S. T. Yoon, PA-C
Thomas A. Zban, MD
Stuart B. Zeilender, MD
Stephanie V. Zeller, MD
Matthew L. Zettl, MD
Shan Zhou, MD
Bradley J. Zins, MD
Jason D. Zook, MD
David A. Zvara, MD
CMS 2024 Virtual Group Election Period Opens October 2

A virtual group is one of several ways clinicians can participate in the Merit-based Incentive Payment System (MIPS). Virtual groups meet the reporting requirements under traditional MIPS; the Alternative Payment Model (APM) Performance Pathway (APP) and MIPS Value Pathways (MVPs) aren’t reporting options available to virtual groups for the 2024 performance year. To receive approval to participate in MIPS as a virtual group for the 2024 performance year, you must submit an election via email ([email protected]) during the virtual group election period, which opens on October 2, 2023, and closes at 11:59 p.m. ET on December 31, 2023.
A virtual group is a combination of 2 or more Taxpayer Identification Numbers (TINs) that elect to form a virtual group for the performance year. There isn't a limit to the number of TINs composing a virtual group. The composition of a virtual group will be one of the following:
- Solo practitioners (who are MIPS eligible).
- Groups consisting of 10 or fewer clinicians (including at least one MIPS eligible clinician).
- Both - solo practitioners (who are MIPS eligible) and groups of 10 or fewer clinicians (including at least one MIPS eligible clinician).
The election process is the same for all virtual groups, regardless of composition.
Here are some things to consider when forming a virtual group:
- TINs participating in MIPS at the virtual group level must meet the definition of a virtual group at all times during the performance year.
- If a group chooses to join a virtual group, all of the clinicians in the group are part of the virtual group.
- A virtual group might include clinicians who are also participating in Advanced APMs.
- Advanced APM participants who achieve Qualifying APM Participant (QP) status will be excluded from MIPS.
A virtual group’s performance is assessed and scored for traditional MIPS at the virtual group level across all 4 MIPS performance categories (quality, cost, improvement activities, and Promoting Interoperability). Virtual groups must aggregate the performance data for all clinicians (across the multiple TINs) within the virtual group for the quality, improvement activities, and Promoting Interoperability performance categories. A virtual group doesn’t need to submit data for the cost performance category because there are no data submission requirements for the cost performance category. We use administrative claims data to calculate a score for the cost performance category on behalf of all clinicians in the virtual group.
Each clinician (National Provider Identifier (NPI)) who is part of a virtual group will receive a final score based on the performance of the virtual group. However, only clinicians who are MIPS eligible will receive a MIPS payment adjustment based on the virtual group’s final score, regardless of any data that may be submitted at the individual, group, or APM Entity level.
Data Collection and Submission Types
There are multiple ways to report data to CMS as a virtual group. The way in which you collect and submit data to CMS depends on:
- The size of the virtual group.
- The type of information technology you use.
- The performance category you’re reporting.
Virtual groups should consider which collection and submission type(s) best fit their virtual group. Virtual groups are encouraged to review the available measures and activities under each collection type(s) to determine which collection type to use and then select a submission type that supports the measures and activities the virtual group would like to report.
Most collection and submission types are available to virtual groups; however, there's an exception specific to reporting for the quality performance category – Medicare Part B claims can only be used to report quality measures by virtual groups with the small practice designation (i.e., 15 or fewer clinicians in the virtual group).
To learn more about virtual groups, review the resources in the 2024 Virtual Group Toolkit (ZIP, 2MB). [source]
NC Medicaid: Hospital at Home Program Re-Launching
During the Public Health Emergency, the Centers for Medicare and Medicaid Services (CMS) instituted the Hospital Without Walls initiative, known in North Carolina as the Acute Hospital Care at Home (HAH) program. Acute HAH was made available temporarily during the early COVID-19 surge as a Medicaid tool to provide relief to hospitals with limited bed capacity. This coverage ended on March 31, 2021.
The Department underwent an extensive evaluation of that program to inform future coverage through an External Quality Review Organization (EQRO). The findings from that study will be released publicly and show mixed results compared to other studies of Hospital at Home programs since the inception of this model.
There are several factors that result in a more positive outcome with Hospital at Home programs, including a key tenet of successful implementation is the capacity building time required for a hospital to establish the best practices that lead to the best outcomes. The hospitals under the current CMS waiver have been working on their programs since the program evaluation completed March 31, 2021, and have provided assurances that their outcomes are equal to that or better than other HAH evaluations nationally.
After extensive discussions with CMS, NC health systems, NC Medicaid Standard Plans, and other State Medicaid programs, NC Medicaid will cover Acute HAH under the existing DRG methodology from Nov. 1, 2023, until Dec. 31, 2024, when the CMS Medicare Waiver flexibility is scheduled to end.
After that time, if CMS continues to allow this model of care, NC Medicaid anticipates that the reimbursement methodology may shift to a percent of DRG and ultimately into a bundled care value-based care methodology in line with other departmental initiatives to drive population health through shared accountability to outcomes. Future reimbursement details will emerge, and continued coverage past December 2024 will be determined based on a combination of factors including:
- Long term approval by CMS to continue the model after Dec. 31, 2024, and
- Analysis of admissions to Acute Hospital at Home from November of 2023 through October of 2024 and positive findings on the resultant outcomes for Medicaid members.
Effective Nov. 1, 2023, to bill for Acute HAH, providers should bill DRG claims using revenue code 0161 for room and board and occurrence span code 82.
Questions? Contact NCTracks Call Center: 800-688-6696
Duke Health Study Could Advance Research in Blood-Brain Barrier

DURHAM – Up to 40% of older surgery patients develop postoperative delirium, a syndrome of confusion that typically occurs in the first few days after surgery. Postoperative delirium is associated with longer hospitalizations, significant distress, and major postoperative complications, but its underlying causes have been elusive.
Previous studies in mice revealed that postoperative delirium may be due to disruptions of the blood-brain barrier, a layer of cells in the brain’s blood vessels that prevent substances from entering the brain. Large human studies in this area had not been possible due to technical challenges in assessing the barrier. A new Duke Health study has overcome these challenges and is one of the first large studies to demonstrate that patients who develop postoperative delirium have increased openings in the blood-brain barrier.
The study, published in Annals of Neurology, also found that patients who had lower performance on cognitive tests prior to surgery were more likely to experience postoperative delirium.
“These findings are significant because we now have a roadmap to tackle a problem that impacts millions of older adults in the U.S. every year,” said Michael Devinney, M.D., Ph.D., corresponding author of the study, and assistant professor in the Department of Anesthesiology at Duke University School of Medicine.
“If we can find a way to prevent the blood-brain barrier from opening after surgery or figure out what is passing through this barrier and getting to the brain, we can work to develop a therapy that may prevent postoperative delirium,” Devinney said.
To arrive at these findings, scientists examined more than 200 patients, ages 60 or older, before and after non-cardiac and non-neurological surgeries. To understand changes in the blood-brain barrier’s permeability, they collected spinal fluid and blood samples before surgery and 24 hours after, measuring the ratio of a protein called albumin as an indicator of the barrier’s fluidity.
The research team also wanted to control for cognitive ability as a risk factor for delirium. To do so, they administered extensive cognitive evaluations before and after surgery.
They found increased blood-brain barrier permeability is associated with greater delirium rates and longer hospital stays amongst all patients, suggesting that the permeability increase is a response to surgery. They also found that those with lower cognitive abilities experienced increased delirium rates. However, the lower performance on cognitive testing affected delirium risk separate from postoperative blood-brain barrier opening.
“Our work suggests that there may be a two-hit model for postoperative delirium,” Devinney said. “It appears to involve both a predisposing factor such as impaired preoperative cognition, and a precipitating factor involving postoperative blood-brain barrier dysfunction.”
It is known that inflammatory molecules can pass through a leaky blood-brain barrier and cause cognitive impairments. Devinney said researchers are already planning a follow-up study to take a closer look at the collected spinal fluid samples to see if particular inflammatory factors are present in higher concentrations amongst patients who experienced delirium. [source]
NCMS Joining with Other Organizations on HB560, the Diagnostic Imaging Parity Bill, As We Focus on 2024
One bill that the NCMS has been advocating for during the 2023 legislative session is HB560 / Diagnostic Imaging Parity. This bill would increase access to medically necessary diagnostic breast imaging by eliminating burdensome out-of-pocket costs for medically necessary diagnostic and supplemental imaging.
HB560 passed the House of Representatives unanimously, 116-0, on May 6, 2023 and has failed to advance in the Senate. With the 2023 session winding down, advocacy efforts on this bill are now focused on 2024. To set the stage for its consideration, the NCMS has joined with other partner organizations in a letter encouraging favorable consideration by the Senate.
A copy of the letter can be accessed here.
TBT: Celebrating 20 years of Leadership in Medicine! Here is Dr. Vinay Saranga's MEDTalk from 2019
The North Carolina Medical Society is celebrating 20 years of Leadership in Medicine!
Here is a Throwback Thursday to Vinay Saranga, MD's MEDTalk from 2019 on "Smoking Cessation Training"
Look for a new TBT every Thursday until the 2023 LEAD Conference and Golden Stethoscope Gala, October 13-14.
https://youtu.be/oRL--8Cd1Ns
The 2023 LEAD conference registration is OPEN.
Click here for more information.
Please contact Erica Hall at NCMS to organize a class table at the LEAD Gala of your own.
NC Medicaid: New Billing Guidelines for Risperidone Extended-Release Injectable Suspension

Effective with date of service May 26, 2023, the NC Medicaid programs cover risperidone extended-release injectable suspension, for subcutaneous use (Uzedy) for use in the Physician Administered Drug Program (PADP) when billed with HCPCS code J3490 - Unclassified drugs.
Strength/Package Sizes: Extended-release injectable suspension: 50 mg/0.14 mL, 75 mg/0.21 mL, 100 mg/0.28 mL, 125 mg/0.35 mL, 150 mg/0.42 mL, 200 mg/0.56 mL, and 250 mg/0.7 mL single-dose prefilled syringes
Indicated for the treatment of schizophrenia in adults.
Recommended Dose: To start Uzedy, switch from oral daily risperidone. Initiate Uzedy, as either a once monthly injection or a once every two month injection, the day after the last dose of oral therapy. Neither a loading dose nor supplemental oral risperidone doses are recommended when switching.
Dosage recommendations for switching from daily oral Risperidone to Uzedy:
Option 1: Uzedy dosage once monthly
- Prior Therapy = 2 mg of oral risperidone per day: 50 mg
- Prior Therapy = 3 mg of oral risperidone per day: 75 mg
- Prior Therapy = 4 mg of oral risperidone per day: 100 mg
- Prior Therapy = 5 mg of oral risperidone per day: 125 mg
Option 2: Uzedy dosage once every two months
- Prior Therapy = 2 mg of oral risperidone per day: 100 mg
- Prior Therapy = 3 mg of oral risperidone per day: 150 mg
- Prior Therapy = 4 mg of oral risperidone per day: 200 mg
- Prior Therapy = 5 mg of oral risperidone per day: 250 mg
Patients can switch between doses of Uzedy once monthly and once every two months by administering the first dose of the new dosing regimen on the next scheduled date of administration in the original dosing regimen. Revise the dose administration schedule to reflect the change.
See full prescribing information for further detail.
For Medicaid Billing
- The ICD-10-CM diagnosis codes required for billing are:
- F20.0 - Paranoid schizophrenia;
- F20.1 - Disorganized schizophrenia;
- F20.2 - Catatonic schizophrenia;
- F20.3 - Undifferentiated schizophrenia;
- F20.5 - Residual schizophrenia;
- F20.89 - Other schizophrenia
- Providers must bill with HCPCS code: J3490 - Unclassified drugs
- One Medicaid unit of coverage is: 0.5 mg
- The maximum reimbursement rate per unit is: $13.30560
- Providers must bill 11-digit NDCs and appropriate NDC units. The NDCs are: 51759-0305-10, 51759-0410-10, 51759-0520-10, 51759-0630-10, 51759-0740-10, 51759-0850-10, 51759-0960-10
- The NDC units should be reported as "UN1"
- For additional information, refer to the January 2012, Special Bulletin, National Drug Code Implementation Update.
- For additional information regarding NDC claim requirements related to the PADP, refer to the PADP Clinical Coverage Policy 1B, Attachment A, H.7 on DHB's website.
- Providers shall bill their usual and customary charge for non-340B drugs.
- PADP reimburses for drugs billed for Medicaid beneficiaries by 340B participating providers who have registered with the Office of Pharmacy Affairs (OPA). Providers billing for 340B drugs shall bill the cost that is their actual acquisition cost. Providers shall indicate that a drug was purchased under a 340B purchasing agreement by appending the "UD" modifier on the drug detail.
- The fee schedule for the PADP is available on the NC Medicaid Fee Schedule & Covered Code portal.
ICD-10-CM Manual. American Medical Association, 2023 manual.
*Information current as of June 14, 2023, and is not a substitute for professional judgment. For full prescribing information, please refer to current package insert or other appropriate sources prior to making clinical judgments.
For questions, contact: NCTracks Call Center, 800-688-6696
Wake County Medical Society: Proposed Bylaws Update for 2023
WCMS BYLAWS – PROPOSED REVISION 2023
The Wake County Medical Society observes 120 years of existence in 2023. In recent years it has undergone significant changes in composition and function. Our current Bylaws were last revised in 2006. The Executive Council (EC) felt that a revision of the WCMS Bylaws is necessary to reflect these changes and to facilitate efficient future operation. Accordingly, our Bylaws have been reviewed, rewritten, and streamlined by the EC to meet this objective, pending approval by the members in good standing of the Society. The role of Dr. David Gremillion should be recognized as leading this process with the input of the Executive Council. Our goal is to maintain a relevant and independent local medical society.
These proposed new Bylaws have been unanimously approved by the Executive Council. They were announced as a first reading at the general meeting held at the NCMS Building on June 20, 2023. Because of the length of the document, the draft is being distributed electronically to the membership who are encouraged to read the document and respond if questions or recommendations. It will then be presented for a second reading at the general meeting scheduled October 17, 2023, at which time it is planned that a vote of approval will occur.
While our 2006 Bylaws do not specify a quorum, state law requires that 10% of members in good standing (Active and Life) respond in person or by proxy. Thus, you are strongly encouraged to attend and participate to support the future of your WCMS.
Explanation of the most significant changes:
The current Bylaws revised in 2006 (OLD) are 19 pages in length while the proposed (NEW) are 13 pages in length. This is reduction due largely to deletion of chapters and sections which are no longer relevant or which will be addressed by Policy, allowing greater flexibility.
NEW Chapter 1 describes our Name and Operating Principles, and our 501 (C)3 status.
NEW Chapter 2 Purposes is basically a mission statement.
NEW Chapter 3 Membership corresponds with OLD Chapter 1 Membership in the Society. It clarifies membership categories, defining criteria for Life membership status and specifying that Life status must be requested by the member and approved by the Executive Council rather than just occur automatically. This is important for the accuracy of our records. Also “members in good standing” are defined as being current with dues payments unless they are Life members who are not obligated to pay dues. The OLD Sec 1.3 application for membership process will be addressed in Policy.
The OLD Sec 1.8. Rules of Conduct and Discipline are deleted. These functions are appropriately addressed by the NC Medical Board rather than the WCMS.
OLD Chapter 2. Powers and Duties deleted.
OLD Chapter 3. Meetings addressed in NEW Chapter 7.
OLD Chapter4. Election of Officers. The position of President Elect is eliminated as unnecessarily restrictive and redundant. Circumstances may allow individuals with other experience to fill this role. The NEW Bylaws call for two-year terms and allow successive terms of office. These changes are made to address leadership succession and improve leadership continuity.
OLD Chapter 5. Duties of Officers. These are described in NEW Chapter 5. The Secretary and Treasures are described as separate roles in the NEW Bylaws but may be held by one individual as is currently the case.
NEW Section 5.6 describes the duties of an Executive Director but allows the Society to contract with an organization such as NCMS for services rather than employ an individual.
OLD and NEW Chapter 6 addresses the duties of the Executive Council.
OLD Chapter 6 specifies many committees, most of which are deleted because no longer functional. Non-standing committees may be created. See NEW Chapter 9
Chapter 7 addresses the conduct of meetings.
NEW Chapter 8 describes voting and describes a quorum as required by state law.
NEW Chapter 9 specifies only 3 standing committees – Nominating, Finance, and Program as well as permitting other non-standing committees.
OLD Chapter 9 Amendments replaced by NEW Chapter 12.
OLD Chapter 10 Conflict of Interest replaced by NEW Chapter 11 which will refer this to policy.
Help Our Feathered Friends. Go Dark Tonight and Tomorrow Night.

Tonight and tomorrow are the busiest nights of the year for bird migration over North Carolina. Each year, hundreds of millions of birds die by flying into buildings (even average height buildings) because they are confused by their lights at night.
The Audubon Society's Lights Out program is a national effort to reduce bird-building collisions. The program encourages building owners and managers to turn off, shield, or dim unnecessary lighting during spring and fall migration. The goal is to provide safe passage for migrating birds as they fly between their nesting and wintering grounds.
Light pollution can disorient birds and cause them to crash into buildings. Many birds are killed or injured in collisions with buildings or drop from exhaustion after circling them.
The Audubon Society encourages people to turn off excess lighting, especially outdoors, during migration. If you can't turn off your indoor lights, closing blinds or curtains will also help.
Novant Health Cruises into Better Healthcare
Novant is taking healthcare on the road.
CHARLOTTE — Bringing health care to those that need it -- that's what Novant Health's second Community Care Cruiser aims to do. A celebration was held Monday as the new hospital room on wheels hit the road.
Novant Health, in partnership with Truist, revealed a brand-new Community Care Cruiser to improve access to care for patients in the Charlotte area and provide back-to-school immunizations to Charlotte-Mecklenburg Schools students.
Continue to the full article here.
Eligible participants are children, newborn to age 18. The cruiser is designed to meet the needs of the uninsured youth in the greater Charlotte area. Those who have full insurance coverage should continue to see their primary care physician.
Step 1: There are two ways to make an appointment. Call 844-644-3578 (NHHELPU) or visit the online appointment scheduler.
Step 2: Please email or text a copy of the child’s immunization records to [email protected] or 704-351-1703 before the appointment date. Sending this information in advance will drastically reduce wait time.
For more information about the Community Care Cruiser, call 844-644-3578 (NHHELPU).
NC Medicaid: Updated Policy for Behavioral Health Clinicians

Effective September 15, 2023, the following technical changes were made to:
Intensive In-Home (IIH)
Staffing requirements (a): removed “effective date of this policy. For associate level licensed team leaders hired after the effective date of this policy, the 30-month timeline begins at.” The amended language now indicates, “An associate level professional actively seeking licensure may serve as the team leader conditional upon being fully licensed within 30 months from the date of hire.”
Psychosocial Rehabilitation (PSR)
Eligibility Criteria (b): “Level of Care Criteria are met” language removed.
Child and Adolescent Day Treatment (DT)
- Staffing Requirements (d): “dedicated” removed as requirement for licensed staff to allow flexibility for staffing. The removal of this language does not reduce staffing requirements.
- Staffing Requirements (d): removed “effective date of this policy. For associate level licensed professionals hired after the effective date of this policy, the 30-month timeline begins at.” The amended language now indicates, “An associate level licensed professional who fills this position must be fully licensed within 30 months from the date of hire.”
Partial Hospitalization (PH)
- Eligibility Criteria (a): "or substance use disorder" removed as an eligibility criterion to align with the Medicaid State Plan Amendment and 10A NCAC 27 g .1100.
- Eligibility Criteria (b): "Level of Care Criteria" language removed.
Effective October 1, 2023, a technical change was made to Attachment A: Claims-Related Information (C.)
Codes): Telehealth Eligible column checked for CPT 96121.
For questions, contact NC Medicaid Contact Center: 888-245-0179
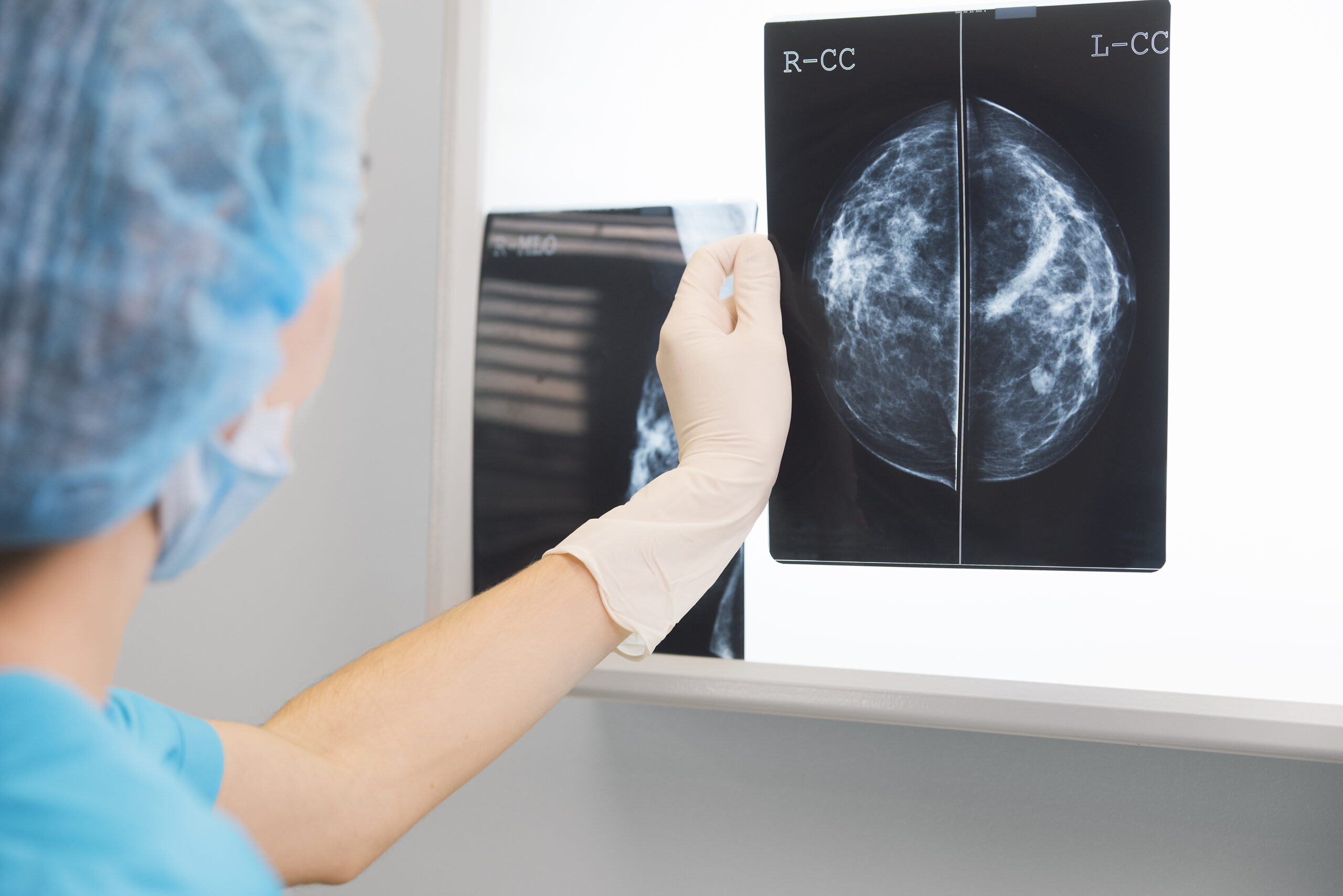
UNC Researchers Develop AI Model to Improve Tumor Removal Accuracy During Breast Cancer Surgery
Artificial intelligence (AI) and machine learning tools have received a lot of attention recently, with the majority of discussions focusing on proper use. However, this technology has a wide range of practical applications, from predicting natural disasters to addressing racial inequalities and now, assisting in cancer surgery.
A new clinical and research partnership between the UNC Department of Surgery, the Joint UNC-NCSU Department of Biomedical Engineering, and the UNC Lineberger Comprehensive Cancer Center has created an AI model that can predict whether or not cancerous tissue has been fully removed from the body during breast cancer surgery. Their findings were published in Annals of Surgical Oncology.
“Some cancers you can feel and see, but we can’t see microscopic cancer cells that may be present at the edge of the tissue removed. Other cancers are completely microscopic,” said senior author Kristalyn Gallagher, DO, section chief of breast surgery in the Division of Surgical Oncology and UNC Lineberger member. “This AI tool would allow us to more accurately analyze tumors removed surgically in real-time, and increase the chance that all of the cancer cells are removed during the surgery. This would prevent the need to bring patients back for a second or third surgery.”

During surgery, the surgeon will resect the tumor (also referred to as a specimen) and take a small amount of surrounding healthy tissue in an attempt to remove all of the cancer in the breast. The specimen is then photographed using a mammography machine and reviewed by the team to make sure the area of abnormality was removed. It is then sent to pathology for further analysis.
The pathologist can determine whether cancer cells extend to the specimen’s outer edge, or pathological margin. If cancer cells are present on the edge of the tissue removed, there is a chance that additional cancer cells still remain in the breast. The surgeon might have to perform additional surgery to remove additional tissue to ensure the cancer has been completely removed. However, this can take up to a week after surgery to process fully, while specimen mammography, or photographing the specimen with an X-ray, can be done immediately in the operating room.
To “teach” their AI model what positive and negative margins look like, researchers used hundreds of these specimen mammogram images, matched with the final specimen reports from pathologists. To help their model, the researchers also gathered demographic data from patients, such as age, race, tumor type, and tumor size.

After calculating the model’s accuracy in predicting pathologic margins, researchers compared that data to the typical accuracy of human interpretation and discovered that the AI model performed as well as humans, if not better.
“It is interesting to think about how AI models can support doctor’s and surgeon’s decision making in the operating room using computer vision,” said first author Kevin Chen, MD, general surgery resident in the Department of Surgery. “We found that the AI model matched or slightly surpassed humans in identifying positive margins.”
According to Gallagher, the model can be especially helpful in discerning margins in patients that have higher breast density. On mammograms, higher density breast tissue and tumors appear as a bright white color, making it difficult to discern where the cancer ends and the healthy breast tissue begins.
Similar models could also be especially helpful for hospitals with fewer resources, which may not have the specialist surgeons, radiologists, or pathologists on hand to make a quick, informed decision in the operating room.

“It is like putting an extra layer of support in hospitals that maybe wouldn’t have that expertise readily available,” said Shawn Gomez, EngScD, professor of biomedical engineering and pharmacology and co-senior author on the paper. “Instead of having to make a best guess, surgeons could have the support of a model trained on hundreds or thousands of images and get immediate feedback on their surgery to make a more informed decision.”
Since the model is still in its early stages, researchers will keep adding more pictures taken by more patients and different surgeons. The model will need to be validated in further studies before it can be used clinically. Researchers anticipate that the accuracy of their models will increase over time as they learn more about the appearance of normal tissue, tumors, and margins. [source]
What Is Your Burnout Risk? How Long You’ve Been in Practice Can Tell A Tale.

Attention has long been focused on the challenges faced by newly minted physicians as they navigate the intricate maze of medicine. But physician burnout, poor job satisfaction and other professional challenges vary by career stage. And middle career appears to be an especially challenging time for physicians, according to an exclusive survey from the AMA. The results signal a pressing need to tailor efforts to promote physician satisfaction, reduce burnout and improve retention based on where doctors are in their careers.
More than 13,000 responses from physicians and nonphysician providers across 30 states were received from more than 70 health systems that participated in the AMA’s Organizational Biopsy® (PDF). The AMA benchmarking report—which is exclusive data to the AMA that is not published anywhere else—reflects 2022 trends in six key performance indicators: job satisfaction, job stress, burnout, intent to leave an organization, feeling valued by an organization, and total hours spent per week on work-related activities (known as “time spend”).
Continue to full article and delve into the AMA data and what it reveals about physician burnout rates here.
NCMS LEAD MedTalk Speaker Showcase: Susie Fitzgerald, DO
NCMS Welcomes 2023 Leadership College Scholar
Susie Fitzgerald, DO
Join us at the 2023 LEAD Conference and Golden Stethoscope Awards Gala Next Month!
Hear Dr. Fitzgerald's MedTalk: "Implementing Screening for Social Determinants of Health in Rural North Carolina"
To learn more about leadership training opportunities at NCMS click here.
To register for the 2023 LEAD Conference and Golden Stethoscope Awards Gala or to become a sponsor click here.
Medicine is Changing. Have Thoughts About It? Let YOUR Voice be Heard in the NCMS On Point Blog!
Submit Your On Point Editorial
The NCMS Communications Team is looking for Medical Society members to contribute to the “On Point” blog and Podcast. Submissions to the “On Point” blog must meet the following criteria:
- Focus on a current or emerging issues that (a) are of interest to our member—physicians and physician assistants; (b) focus on chronic health, mental health, or integrated health topics; or (c) opinion pieces about lifestyle and wellness topics.
- Blog submissions must be 500-1,000 words for decent depth and detail.
- Podcasts are 30 to 60 minutes in length and will be interview format or can be an original pre-recorded discussion submitted as a mp4 format file.
- ALL content must be original content of the author and does not include self-promotion or marketing information.
- All submissions must be well-written and complete, no outlines.
- Submissions are restricted to NCMS members only.
Please send submissions to [email protected].
The blog is for educational and informational purposes.
NC to Receive More than $4 Million to Address Maternal Health

RALEIGH — The North Carolina Department of Health and Human Services is pleased to be selected among several entities for the U.S. Department of Health and Human Services’ Health Resources and Services Administration investment in increasing access to maternal care, addressing maternal mental health and growing the maternal health workforce in North Carolina. The department is committed to addressing the maternal mortality crisis and ongoing disparities among infant and maternal mortality rates, particularly in underserved areas. Black babies in North Carolina are 2.5 times more likely to die than white babies, and Black women experience almost twice the rate of maternal mortality than white women.
"Every mother deserves to have a healthy pregnancy and every baby deserves to have a healthy start in life," said NCDHHS Secretary Kody H. Kinsley. "This investment from HRSA will provide more resources to address the disparities in maternal health including a focus on maternal behavioral health, so we can ensure mothers and families get the care they need, when they need it before, during and after pregnancy."
More than $1.1 million each year for five years will go towards the Healthy Start Initiative in the department’s work towards eliminating racial and ethnic disparities. The funding will focus on Cumberland and Hoke counties due to their high Black infant mortality rate. Eighty percent of health outcomes are influenced by factors outside of the health care system. Healthy Start has a specific emphasis on addressing those factors, like housing and nutrition, in order to improve disparities and infant health outcomes. The program will provide direct community support for people who need it and will serve people before, during and after birth, along with fathers, infants and children up to 18 months old.
Funds will also be used for the screening and treatment of maternal depression and related behavioral health disorders in the NC Maternal Mental Health MATTERS Program. The 5-year funding for $750,000 will elevate the work by staffing NC-PAL's perinatal health component of the line and offer clinical assessments through NCDHHS partnerships with Duke, UNC and other providers. The program includes provider education to support and train OB/GYNs, midwives and other maternal health providers in treating mental health and substance use disorders.
Following the grant announcements, NCDHHS Secretary Kody H. Kinsley, NCDHHS Section Chief for Women, Infant and Community Wellness Belinda Pettiford and other NCDHHS leadership participated in a roundtable discussion with HRSA Administrator Carole Johnson and Dr. Michael Warren with HRSA on Thursday at the Wake County Human Services.
More information on the funds NCDHHS and other North Carolina partners are receiving is available in the press release from HRSA. [source]
New CMS Value-Based Care Model for North Carolina Primary Care Practices

The Centers for Medicare and Medicaid Services (CMS) has announced a new voluntary value-based primary care model called Making Care Primary (MCP). With a planned launch date of July 1, 2024 in eight states including North Carolina, the 10.5-year model aims to improve care management and care coordination, equip primary care clinicians with tools to form partnerships with health care specialists, and leverage community-based connections to address patients’ health needs.
CMS is now accepting applications from Medicare-enrolled organizations that provide primary care services to fee-for-service Medicare beneficiaries. The application deadline is November 30, 2023.
NCAHEC has developed a playbook for potential applicants, which contains concepts about the MCP model and its design, timelines, evaluation tools, considerations, and resources. It's a must-see for those interested in applying!
For more information, visit CMS.
Take Precautions! West Nile Virus Cases on the Rise in North Carolina.
Early fall is one of the most common times to become infected with mosquito-borne diseases.
NCDHHS urges North Carolinians to take precautions.

RALEIGH — The North Carolina Department of Health and Human Services reminds people to take precautions against mosquito bites following recent reports of West Nile virus infection in the state. Late summer and early autumn are the most common time to become infected with mosquito-borne diseases in North Carolina like WNV.
Five cases of WNV have been reported in North Carolina residents since Aug. 1. All individuals have recovered, and to protect their privacy, no other information will be released. Typically, by this time of year, only two or three WNV cases have been reported on average.
"As we move into the fall months and colder weather, this is a reminder that mosquitos are still active and can transmit West Nile virus," said Michael Doyle, State Public Health Entomologist. "We urge residents to take precautions and protect themselves from mosquito bites and local governments to implement integrated mosquito management methods for mosquito control."
According to the Centers for Disease Control and Prevention, most people who become infected with WNV do not develop any symptoms. About one in five people who are infected develop a fever with other symptoms such as headache, body aches, joint pains, vomiting, diarrhea or rash. About one in 150 people who are infected develop a severe illness affecting the central nervous system such as encephalitis (inflammation of the brain) or meningitis (inflammation of the membranes that surround the brain and spinal cord).
West Nile is not the only mosquito-borne virus in the state. Approximately 20 cases of La Crosse encephalitis are reported each year in the western part of the state, and one case has been reported so far in 2023. There is also always a small risk of contracting eastern equine encephalitis in the eastern counties, but no cases have been reported this year.
NCDHHS recommends individuals take the following precautions to protect against mosquito bites and mosquito-borne illness:
- Use mosquito repellent that contains DEET (or equivalent) when outside in areas where mosquitoes might be present.
- Use caution when applying repellent to children. See www.epa.gov/insect-repellents/find-repellent-right-you for repellants that will work for you and your family.
- Install or repair and use window and door screens.
- Close doors without screens, including garage doors. Do not leave doors propped open.
- Use air conditioning if needed due to a lack of window or door screens.
- Reduce mosquito breeding by emptying standing water from flowerpots, gutters, buckets, pool covers, pet water dishes, discarded tires, birdbaths, etc. at least once a week.
- If you think you or a family member might have WNV disease, talk with your health care provider.
Local, state and federal public health agencies work throughout the year to investigate and respond to cases of mosquito-borne infections, educate the public on preventing mosquito bites and support local mosquito management programs. [source]
For more information on West Nile virus and the prevention of mosquito bites visit epi.dph.ncdhhs.gov/cd/diseases/wnv.html and www.cdc.gov/westnile/faq/repellent.html.
CDC Advisory Committee Recommendations on Flu Vaccination for People with Egg Allergies
September and October are good times to get a flu vaccine.

In June, the Center for Disease Control and Prevention (CDC) adopted the 2023-2024 Advisory Committee on Immunization Practices’ (ACIP) recommendations on annual influenza (flu) vaccination for everyone 6 months and older in the United States. The main change in the flu vaccine recommendations is related to giving flu vaccine to people with egg allergies.
Most flu vaccines today continue to be produced using an egg-based manufacturing process and therefore contain a small amount of egg proteins, such as ovalbumin.
While ACIP has previously recommended that all people 6 months and older with egg allergy should be vaccinated for flu, in the past there have been additional safety measures recommended for administration of egg-based flu vaccine to people who have had severe allergic reactions to egg. The ACIP voted that people with egg-allergy may receive any flu vaccine (egg-based or non-egg based) that is otherwise appropriate for their age and health status. Additional safety measures are no longer recommended for flu vaccination beyond those recommended for receipt of any vaccine. [source]
Plan Now! Preventing Gender-Based and Intimate Partner Violence Webinar in October

Join the Office of Women's Health (OWH), the Bureau of Health Workforce, and the Director of Sexual and Gender-Based Violence within the HHS Office of the Assistant Secretary for Health for a webinar, Preventing Gender-Based and Intimate Partner Violence.
It will be held Thursday, October 12, 2-3:00 p.m. ET, in observance of Domestic Violence Awareness Month. Register to learn about HRSA and HHS programs that address gender-based and intimate partner violence.
Download HRSA OWH's newly released Implementation Framework for Preventing and Responding to IPV.
Nominating and Leadership Development Committee Issues Final Slate of Nominees

The North Carolina Medical Society (NCMS) Nominating and Leadership Development Committee issues the final slate of candidates.
Under the NCMS’ governance structure, online voting for the candidates will begin on September 28, 2023, and continue until midnight on October 12, 2023. Paper ballots will also be available to those who need them.
The following slate of nominees was selected by the Nominating and Leadership Development Committee:
NCMS Board of Directors
President Elect: John Meier, IV, MD
Secretary-Treasurer: Tracy Eskra, MD
Region 3 Representative: Karen Smith, MD
Region 4 Representative: Martin Palmeri, MD
At-Large Member: Bryant Murphy, MD
At-Large Member: William Ferrell, MD
At-Large Member: Ronald Laney, Jr., MD
NC American Medical Association Delegation
AMA Delegate: Mary Ann Contogiannis, MD
AMA Delegate: Justin Hurie, MD
Nominating and Leadership Development Committee
NLDC Region 2: John Chiavetta, MD
NLDC Region 2: Damian McHugh, MD
NC Medicaid: Complete All EVV Data to Avoid Claim Denials

Starting Oct. 1, 2023, the North Carolina Department of Health and Human Services (NCDHHS) will implement a hard launch of the Electronic Visit Verification (EVV) system for Home Health Care Services (HHCS) rendered under both the Standard Plans and NC Medicaid Direct.
EVV will verify:
- Date of service
- Beneficiary receiving services
- Location of service
- Individual providing service
- Type of service rendered
- Time the service begins and ends
Services covered through the State Plan and the 3-A Home Health Clinical Coverage Policy that will be subject to the EVV requirement:
- Home health aide
- Physical therapy
- Speech therapy
- Occupational therapy
- Skilled nursing visits
On this date:
- All home health providers are expected to be fully compliant with EVV requirements
- EVV data must be validated prior to claims adjudication, and
- Claims without the required EVV criteria will be denied.
For all question, contact: [email protected]
Free Covid-19 Tests Returning Monday!

“We will once again begin our program to provide Americans with an opportunity to request tests,” HHS Secretary Xavier Becerra said during an event at a Washington CVS Pharmacy where he was vaccinated against Covid-19 and flu.
US households can order four free tests from Covidtests.gov starting September 25.
The US government has shipped more than 755 million free Covid-19 tests to people who requested them through Covidtests.gov. The program was suspended in May, after the end of the Covid-19 public health emergency, to preserve supply.
Becerra said HHS’s Administration for Strategic Preparedness and Response “has been resupplying our stockpile.”
The tests coming available soon are intended for use through the end of 2023 and will include instructions on how to verify extended expiration dates, HHS said in an announcement Wednesday. The expiration dates of many home Covid-19 tests have been extended well beyond what’s printed on the package.
HHS and ASPR also announced $600 million for 12 US Covid-19 test manufacturers to strengthen manufacturing capacity and purchase about 200 million over-the-counter Covid-19 tests for use by the federal government. The funding will go to manufacturers in New Jersey, California, Texas, Washington, Maryland, Pennsylvania and Delaware.
“The Biden-Harris Administration, in partnership with domestic manufacturers, has made great strides in addressing vulnerabilities in the U.S. supply chain by reducing our reliance on overseas manufacturing,” Becerra said in a statement. “These critical investments will strengthen our nation’s production levels of domestic at-home COVID-19 rapid tests and help mitigate the spread of the virus.”
Covid-19 hospitalizations have been on the rise in the United States since July, with weekly admissions now more than triple what they were two months ago. More than 20,500 people in the US were admitted to the hospital with Covid-19 during the week ending September 9, according to data from the US Centers for Disease Control and Prevention — about 8% higher than the week before. [source]
Get Ready! 2024 ICD-10 Codes Update Effective Soon

The 2024 ICD-10 update is effective October 1, 2023, through September 30, 2024, for provider use for dates of service within this period. Providers can access the list of ICD-10 codes on the Centers for Medicare and Medicaid Services (CMS) website.
Click below for new, end dated or revised 2024 ICD-10-CM and ICD-10-PCS codes.
For CM Codes (Diagnosis Codes)
Visit the CMS 2024 ICD-10-CM webpage
- Click on “2024 Code Descriptions in Tabular Order [ZIP]”
- Select the file titled “icd10cm_order-addenda_2024.txt”
For PCS Codes (Procedure Codes)
Visit the CMS 2024 ICD-10-PCS webpage
- Click on “2024 ICD-10-PCS Order File (Long and Abbreviated Titles) [ZIP]”
- Select the file titled “order_addenda_2024.txt”
Have questions, contact NCTracks Contact Center: 800-688-6696
NCMS PAC Thankful Thursday!

On this Thankful Thursday, we are recognizing some of our NCMS PAC Investors! Thank you for your continued investment in your patients and profession. Contact Hannah Rice ([email protected]) to learn more about how you can make a difference.
Gregory M. Swank, MD, FACS
Marty A. Baker, MD
Pankaj Gupta, MD, FACS
Robert E. Schaaf, MD, FACR
Residents and Students: Deadline Extended to Submit Your Scientific Poster for the LEAD Conference
RESIDENTS AND STUDENTS: Share your clinical research or vignettes at the North Carolina Medical Society LEAD Conference
Deadline extended to October 4th!

The North Carolina Medical Society (NCMS) is issuing a Call for Scientific Posters for its LEAD Health Care Conference, scheduled for October 13-14, 2023, at the Sheraton Imperial Hotel - RTP. We encourage all of our up-and-coming physicians to participate in this educational program by submission of a formal application for scientific presentation.
The Poster Session is divided into two categories:
Clinical Research and Clinical Vignette.
Monetary prizes available! All accepted Poster Session participants will be able to attend the LEAD Conference for free! Get all of the details on the LEAD website.
Ready to submit your poster?
For more information or questions regarding the Poster Session, please contact Megan Eberle, [email protected]
TBT: Celebrating 20 years of Leadership in Medicine! Here is Elyse Watkins, PA-C and Christen MacKorell, PA's MEDTalk from 2016
The North Carolina Medical Society is celebrating 20 years of Leadership in Medicine!
Here is a Throwback Thursday to Elyse Watkins, PA-C and Christen MacKorell, PA's MEDTalk from 2016 on "Perceived Barriers to the Utilization of Physician Assistants in OB-GYN Practices in North Carolina"
Look for a new TBT every Thursday until the 2023 LEAD Conference and Golden Stethoscope Gala, October 13-14.
https://youtu.be/cptwm_DI78E
The 2023 LEAD conference registration is OPEN.
Click here for more information.
Please contact Erica Hall at NCMS to organize a class table at the LEAD Gala of your own.
NCTracks: Avoid Delays, Issues, Additional Fees with Your Reverification Application

To prevent delays and problems with processing reverification applications, clinicians should thoroughly review their record in NCTracks prior to submission, including taking the following actions to ensure its accuracy:
- Confirm that all active taxonomies are used by the provider. Each service location must have at least one active taxonomy, but some require additional credentials, screenings (i.e. site visit and fingerprinting), or a federal fee as published on the Provider Permission Matrix (PPM). If a taxonomy is no longer in use, end-date it to avoid possible expense and screening.
- Review each active owner and managing employee (ME), being sure to end-date any who are no longer associated with the individual provider or organization, as background checks are required for everyone who is active on the record.
- If incorrect information is displayed in a field that cannot be edited (i.e., active owner/managing employee name, date of birth, social security number), follow the applicable instructions on the NCTracks Helpful Hints page to end-date the line containing the incorrect information and add a new line with that individual’s correct information.
- This can be done through the reverification application.
- If additional assistance is needed, contact the NCTracks Call Center at 800-688-6696 for guidance.
- Confirm that the provider’s license, accreditation, and certifications are not expiring within 30 days of the date the reverification application is submitted, as this will cause the system not to allow submittal.
- Providers have 70 calendar days to submit their reverification application.
- This gives time before or after the 30-day window for submission of the application if review and response is timely.
Submitting applications with inaccurate or invalid data can lead to the application being withdrawn. If the application is withdrawn, a new application must be submitted with the correct information and will require an additional NC Medicaid application fee to be paid.
For help with the reverification process, providers can refer to the Provider Re-credentialing/Re-verification webpage in the NCTracks public facing portal. Providers are also encouraged to review Provider Announcements, User Guides and Frequently Asked Questions.
As a convenience, NC Medicaid offers a list of “Active Provider Re-Verification Due – July 2023 – Dec 2023” dates (updated biannually). The reverification due date displayed is also the suspension date if no action is taken to submit the reverification application and fee(s) under the applicable NPI. If the health plan is suspended, claims payment will stop until the reverification application is submitted.
Providers should review the reverification due date list and frequently check their NCTracks Provider Message Inbox for notifications or the reverification section of the Status and Management page in the NCTracks Secure Portal for the option to reverify.
Have questions, contact NCTracks Call Center: 800-688-6696
ICYMI: NCMB Confirms CME Completed for DEA Mandate, Also Satisfies NC Requirement

In response to questions from licensees and others, NCMB recently clarified that continuing medical education (CME) hours completed to satisfy a new one-time requirement for DEA registrants may be counted towards the existing CME requirement for controlled substances prescribers in North Carolina. NCMB voted at its July Board Meeting to pursue CME rule changes that specify this.
All prescribers who hold valid DEA registrations must complete eight hours of CME in the treatment of opioid use and other substance use disorders to meet the requirements of a mandate approved as part of the federal Medication Access and Treatment Expansion (MATE) Act. On June 27, DEA began asking renewing registrants to certify that they have completed the one-time training requirement.
Physicians and PA licensees of NCMB who prescribe controlled substances must also comply with an existing CME requirement that obligates them to complete a set number of CME hours in controlled substances prescribing topics, including treatment of opioid use disorder. Physicians must earn 3 hours of controlled substances CME every three years and PAs must earn two hours every two years, in accordance with established CME cycles.
NCMB has created a new FAQ stating that licensees may use their DEA-mandated CME credits to meet the NCMB controlled substances requirement. Find it here.
NCDHHS ALERT: Malicious Phishing Attempt Targeting Nursing, Intermediate Care Facilities for Individuals with Intellectual Disabilities

The North Carolina Department of Health and Human Services has identified a malicious phishing attempt where the state government email address of Stacey Crute, ending in “.gov”, has been spoofed to a non-state email address ending in “.com.” Do not respond or take requested action if you receive this email.
Phishing is a technique for attempting to acquire sensitive data, such as bank account numbers, through a fraudulent solicitation in email or on a website, in which the perpetrator masquerades as a legitimate business or reputable person.
The user of this spoofed account has sent fraudulent email messages to nursing facility providers who are required to pay a monthly assessment (a/k/a provider bed tax). The email attempts to falsely convince providers they must change their method of payment from a paper check remittance to the North Carolina Department of Health and Human Services Accounts Receivable to an electronic remittance for an unknown bank account. Providers should NOT follow the instructions of this phishing email.
The proper method of remittance of the monthly provider assessment continues to be a paper check remittance payable to the Division of Health Benefits with “Provider Fee Assessment” in the Memo Field and mailed to either of the below:
Mailing Address:
DHHS Accounts Receivable
2022 Mail Service Center
Raleigh, NC 27699-2022
Overnight Address:
DHHS Accounts Receivable
1050 Umstead Drive
Dorothea Dix Campus
Raleigh, NC 27603
If providers have been targeted by this phishing attempt, we recommend they contact their own Privacy / Security Officer and follow their agency’s protocols for such an attempt.
If providers have sent provider bed tax funds to this external phishing attempt, we recommend they follow the above and alert the Department contact below:
Alicia Hunter – Office of the Controller [email protected]
Lashan Johnson – Office of the Controller [email protected]
NCMS LEAD MedTalk Speaker Showcase: Jennifer Kipp, DPM
NCMS Welcomes 2023 Leadership College Scholar
Jennifer Kipp, DPM
Join us at the 2023 LEAD Conference and Golden Stethoscope Awards Gala Next Month!
Hear Dr. Kipp's MedTalk: "Building a Comprehensive Tobacco Cessation Program for Elective Foot and Ankle Surgery Patients"
To learn more about leadership training opportunities at NCMS click here.
To register for the 2023 LEAD Conference and Golden Stethoscope Awards Gala or to become a sponsor click here.
Health Professional Students to Service Loan Repayment Program Application Now Open

The 2024 National Health Service Corps (NHSC) Students to Service Loan Repayment Program is now open for applications. Final-year medical, nursing, and dental students are eligible to apply for up to $120,000.
Awardees will make a three-year commitment to provide primary care services at NHSC-approved sites in high-need locations. An additional $40,000 supplement has been added for medical students who commit to providing OB/GYN services in a maternity care target area.
Applications will be accepted through 7:30 p.m. ET on Thursday, December 7.
The Wait is Over! 2023 Photo Contest Results are in!
The results of the 2023 NCMS Photo Contest are in!
As with every year, the North Carolina Medical Society welcomed absolutely breathtaking photo entries from our members. In fact, the submissions were so fierce this year, we had to run a secondary round of judging to determine the final contest winner. Here are your winners:
| Overall Winner | John Goldfield, PA-C | Above the clouds |
| Category Winner – Nature | Kathryn Greven, MD | Badwater Sunset |
| Category Winner – North Carolina | Karl S. Chiang, MD, FACR | Jelly Wonder of the World |
| Category Winner – Travel | James Salisbury, MD | Strong Arm |
| Category Winner – Wildlife | Rodger David Israel, MD, MHL | Leopard in a Tree |





FINALISTS
| Charles H. Classen, Jr., MD | The Ghost Ship |
| Steve Dingeldein, MD | North American Nebula |
| P. Mark Gallerani, MD | Velvet-purple Coronet Hummingbird |
| Tony Huggins, MD | Solitude |
| Joel B. Miller, MD | A Break in the Rain |
| Keith V. Nance, MD | Menacing Cloud in the Badlands |
| Vikas Patel, MD | Dreamy Sunrise |
| Robert M. Varnell, MD | Swimming with Mother and Baby Humpback Whales |
SEMI-FINALISTS
| Brian Bowman, MD, PhD | Castle Stalker |
| Jeffrey Coston, DO | Appalachian Sunset |
| Shirish Devasthali, MD | Back window view in Madeira |
| Lawrence Greenblatt, MD, FACP | Patterns of Light in the Scottish Highlands |
| Richard M. Griffin, MD, FACS | Mom Feeding her Babies |
| Blaine Paxton Hall, MHS-CL, PA-C, DFAAPA, Ret. | St. Paul’s Bay |
| Edgar O. Hartle, MD | Sunset with the sparkling clouds over Bird Island, NC |
| Jim Hill, MEd, PA-C Emeritus, DFAAPA | Ethereal White Egret in the Swamp |
| Hampton A. Howell, MD | Storm’s a-comin’ |
| Mark McClure, MD | Grizzly Bear Play Time |
| Marion Mull McCrary, MD, FACP | Mother’s Day Peonies |
| Curtiss Merrick, MD | Snowy Egret |
| David Mertz, MD | Quiet moments in Venice |
| Thomas J. Monaco, Jr., MD | Tuscan Sunrise |
| Leighton A. Raynor, MD | Female Fork-Tailed Katydid |
| Donna M. Richardson, MD, MBA, FCAP | Grand Canyon at Sunrise |
| Thomas Swantkowski, MD | The Survivor |
| Tanya Sue Woodson, PA-C | Home is Where the Heart Is |
| Robert Yapundich, MD | Cascade Sunrise |
HONORABLE MENTIONS
| Yun L. Boylston, MD, MBA, FAAP | Pastoral View with Black Sand Beaches in Heimaey |
| Tracy Eskra , MD, MBA | You are not alone |
| Stephen Ezzo, MD, FAAP | Stone and Wood |
| Lisa M. Gangarosa, MD | On the shore of the Susquehanna |
| Cynthia M. Gary, MPH, PA-C | Water soothes the soul! |
| Steven M. Genkins, M.D. (“Seadoc”) | My Cuttlefish Teacher |
| Jane Girskis, PA-C | Reflection on creek |
| H. Slade Howell, MD | Lunch Time |
| Ismo M. Kaariainen, MD, FACO, FHM | Nightfall, English Harbour, Antigua |
| Christine Khandelwal, DO, MHPE | Icelandic Horse |
| Gautam Khandelwal, MD | Slumber of Icelandic horses |
| Pete Leuchtmann, MD | Kiawah Sand Dunes |
| Katie Lowry, MD | Hatfields and McCoys (Kitty Version) |
| Robert W. Monteiro, MD | Bumps on a log |
| Arnold C. Olegario, MD | Ride The Storm |
| Liston A. Orr, MD | Bonefish moonrise |
| Bob Park, MD | Aspens watching you |
| Danna Park MD, FAAP, FACP | Arno River Reflections |
| Katherine J. Pierce, MD | Before the Storm |
| Demetri T. Poulis, MD, FACS | Praying Mantis (Mantidae), Nags Head, NC |
| Christopher N. Shatley, PA-C | Sunset over Lake James |
| Nadine B. Skinner, MD, FAAFP | Paradise in Blue |
| Robert Starkenburg, MD | Three Cliffs Bay at Low Tide |
| David Tart, MD | A Spectacular Hebridean Sunset |
| Aryanna Thuraisingam | Stranded in the Dust |
| Cheryl Yanuck, MD | Pacific sunset |
Thank you for sharing your beautiful photos with us! Be sure to watch your Morning Rounds for details on the 2024 NCMS Photo Contest.
NCDHHS Hosts ePASS Training to Prepare for Medicaid Expansion Tuesday
Join NC DHHS for a special Community Partners webinar on ePASS.

The webinar will provide an overview and demonstration of North Carolina’s secure self-service website and help attendees learn how to navigate the ePASS system and complete a Medicaid application. There will be an opportunity for questions and answers.
Community Partners ePASS Webinar
Tuesday, September 19
5:00 - 6:30pm
Register
Tips on Caring for Children Before and After a Disaster

As we go through an active hurricane season, experienced floods, and dealt with wildfires, keep in mind disasters are not only stressful events for adults, but also for children.
After a disaster, children may develop symptoms of anxiety, depression, and post-traumatic stress disorder. Unfortunately, emotional stress from a disaster can be even harder on children because they:
- Understand less about the situation;
- Feel less able to control events;
- Have less experience dealing with stressful situations; and
- May not be able to communicate their feelings, such as fear or anxiety.
Children with previous traumas or have a pre-existing mental, emotional, developmental, or behavioral disorder can be particularly vulnerable before a disaster occurs.

Here are ways to help children cope with a disaster:
Before:
- Discuss plans for emergencies and include children in creating emergency supply kits and emergency action plans.
- Have a plan to reunite with family members as children may be separated from their caregivers during an emergency. If family members cannot return to their home, having an alternative meeting location, such as a community center, can help them reunite after a disaster.
- Learn about safety drills taking place in the child's school or early care and education facility from the administrators and safety officials. Since drills vary by state, you can also look on your state’s Department of Education web page. Talk with the child's teacher or early care and education professionals about how to help the child see safety drills as empowering rather than frightening.
After:
- Answer the child’s questions truthfully in a way that they can understand. You can also correct misinformation about the event.
- Set an example for the child by managing stress and returning to normal routine and encouraging them to do the same. Proceeding with a normal daily routine, such as eating meals as a family or returning to school and work, can help reduce stress.
- If the child’s routines and environment are disrupted, and if unable to provide the same consistent care as before, talk about the changes, how long they will last, and what is being done to create routines and structures.
- Give the child opportunities to talk about what they went through, or what they think about it. Encourage to share concerns and ask questions.
- Allow the child to be with a trusted adult who can help them feel safe and calm and give them a sense of hope. Children can handle disruptions in care better if they know they are temporary.
- Limit exposure to media coverage of the disaster and its aftermath. Children who are directly exposed to a disaster can become upset again if they see or hear reminders of what happened.
- Work with teachers and other adults, who see the child in different situations, to share information about how they are coping. [source]
NC Health Officials Encourage North Carolinians to Get Fall COVID-19 Vaccine and Flu Shot
We have the tools to manage COVID-19 so it does not manage us. Vaccines are our most powerful tool to prevent serious illness and death from COVID-19 and other viruses.
Flu and respiratory syncytial virus (RSV) also spread in the fall and winter. Like COVID-19, these viruses cause a respiratory infection that can lead to severe illness and even death, particularly in older adults, young children and those with underlying medical issues.
"Like the annual flu shot, the fall COVID-19 vaccine provides the most up-to-date protection against the COVID-19 virus and will help us prepare for seasonal viruses that disrupt our lives," said Dr. Elizabeth Cuervo Tilson, State Health Director and NCDHHS Chief Medical Officer. "We encourage everyone to talk with a health care provider about all recommended vaccines. Protect your family so you can enjoy the activities, sports, school and time with loved ones this fall and winter."

Health officials recommend everyone 6 months and older get their annual flu shot, ideally before the end of October. This year, for the first time, there is also an RSV vaccine to protect people 60 years and older. In addition, there is a new medication that can protect infants under 8 months and some older babies at increased risk of severe illness with RSV. People should talk to their doctors about whether RSV protection might also be needed for them or their child.
"COVID-19 continues to be a very real risk for many people, and last fall, COVID-19, flu and RSV spread widely at the same time," said Dr. Zack Moore, State Epidemiologist. "Safe and effective vaccines are the best way to protect against these viruses. They are especially important for those at higher risk of complications — people 65 and older, children younger than 5, pregnant women and those with certain medical conditions, like asthma, diabetes and heart disease."
For people with health insurance, most plans will cover the COVID-19 vaccine at no cost. Children who are insured by Medicaid or are uninsured or underinsured can get vaccines at no cost through the Vaccines for Children (VFC) program, which offers vaccines to eligible children through age 18.
The state will also receive a limited supply of free vaccines for uninsured or underinsured adults through the federal Bridge Access Program. These vaccines will be distributed to safety net providers like Community Health Centers and local health departments and to pharmacies participating in the Bridge Access Program.
Providers began pre-ordering the fall COVID-19 vaccines Sept. 8, and supplies could begin coming into the state at the end of this week, with more availability starting next week. With the exception of VFC and the Bridge Access Program, states do not allocate vaccines to providers. Providers and pharmacies must order vaccine supplies directly from manufacturers. We are encouraging providers and pharmacies to order the vaccine so it will be available for their patients and communities.

Flu vaccines are often available at little to no cost at pharmacies, local health departments, doctor offices and other providers throughout the state. For more information on flu vaccines, visit flu.ncdhhs.gov.
For more information about COVID-19 vaccines, visit MySpot.nc.gov, call the CDC-INFO Contact Center at 800-CDC-INFO or visit ncdhhs.gov/LHD to contact a local health department. Read the CDC's full statement.
Healthcare Organizations: Here Are Some Important Cybersecurity Regulation Updates

Health and human service organizations possess an abundance of protected health information (PHI), by the simple nature of their operations. With phishing, smishing, ransomware, and other dangerous cyberattacks on the rise, the threat posed to these organizations has never been higher. As a result, many government agencies—including the U.S. Department of Health & Human Services (HHS), Office of Civil Rights (OCR), and U.S. Food and Drug Administration (FDA)—are developing updated cybersecurity guidance and laws.
The following represent several of the recent, key cybersecurity updates which are important for health and human service organizations to understand:
Enhanced Cybersecurity Focus within OCR
On February 27, 2023, the HHS announced the formation of a new Enforcement Division, Policy Division, and Strategic Planning Division within the OCR. In addition, OCR also renamed its Health Information Policy Division (HIP) to the Health Information Privacy, Data, and Cybersecurity Division (HIPDC) to better reflect the role cybersecurity plays in their operations.
As HHS’ law enforcement agency, OCR is responsible for enforcing 55 civil rights, including the Health Insurance Portability and Accountability Act of 1996 (HIPAA); investigating complaints; conducting compliance reviews; developing policies; promoting regulations; providing technical assistance; and educating the public about federal civil rights, privacy, and conscience laws.
The OCR’s caseload has multiplied in recent years, with a 69% increase in complaints from 2017 to 2022. As of 2022, the OCR received 51,000 complaints—27% alleged violations of civil rights, 7% alleged violations of conscience/religious freedom, and 66% alleged violations of health information privacy and security laws. Furthermore, large breaches of unsecured protected health information (PHI) have increased in recent years, with hacking accounting for 80% of the breaches OCR has experienced.
This recent reorganization of the OCR has been established to provide a more integrated operational structure for civil rights, conscience protections, privacy protections, and cybersecurity protections.
New HIPPA Regulations Surrounding Data Tracking Technologies
On Dec. 1, 2022, the OCR issued a bulletin to highlight the obligations of HIPPA covered entities and business associates under the HIPAA Privacy, Security, and Breach Notification Rules when using online tracking technologies.
Tracking technologies are often used by organizations to collect and analyze information about user interactions with the organization’s websites, apps, etc. HIPPA’s Privacy, Security, and Breach Notification Rules apply when the information collected through tracking technologies includes PHI and prohibits the use of tracking technologies in a manner that would result in impermissible disclosures of PHI.
This new guidance addresses what a tracking technology is as well as how the HIPAA Rules apply to regulated entities’ use of tracking technologies in the following areas:
- Tracking on user-authenticated webpages
- Tracking on unauthenticated webpages
- Tracking within mobile apps
- HIPAA compliance obligations for regulated entities when using tracking technologies
Amended FDA Act Enforcing Cybersecurity in Medical Devices
Given the increased risk of cybersecurity threats to the healthcare sector, the FDA issued an amendment to the Food, Drug, and Cosmetic Act to add section 524B, Ensuring Cybersecurity of Devices on Dec. 29, 2022.
As of March 29, 2023, this act now requires all new medical device applicants to follow the below steps to ensure cybersecurity:
- Submit a plan on how to monitor, identify, and address cybersecurity issues.
- Develop and maintain processes and procedures to provide reasonable assurance that the device is cybersecure.
- Provide a software bill of materials.
- Comply with all other requirements to ensure the device is cybersecure.
Updated Continuing Care Retirement Communities Regulators
Continuing Care Retirement Communities (CCRCs) are now regulated under the Gramm-Leach-Bliley Act (GLBA) cyber laws. This act requires organizations to safeguard sensitive data and explain their information-sharing practices to their customers.
Expanded Data Safeguards from the Federal Trade Commission Applicable to Entities Using Title IV Funds
In December 2021, the Federal Trade Commission (FTC) amended the standards for Safeguarding Customer Information. These cybersecurity requirements went into effect on June 9, 2023. This change impacts organizations using Title IV funds and could have other impacts as the definition of “covered entity” was greatly expanded.
The above represents only a brief overview of several recent cybersecurity updates impacting health and human service organizations. Thoroughly understanding and complying with these, and other applicable regulations, is key to mitigating the risk of dangerous and costly data breaches and cyberattacks. For assistance navigating these complex cybersecurity regulations, organizations should consider aligning with a trusted advisor who can offer tailored guidance specific to an organization. [source]
OTHER HELPFUL RESOURCES:
The American Medical Association (AMA) has curated resources and has tips for physicians and health care staff to protect patient health records and other data from cyberattacks.
In May, the U.S. Food and Drug Administration (FDA) launched its third video focusing on how to prepare for a cybersecurity event and help ensure patient safety during a prolonged cybersecurity event.
EHR Systems: Helpful or Distracting?

Research from the University of Utah Health system shows the importance of ease-of-use for physicians. Their findings suggest that Electronic Health Records (EHR) that are difficult to use result in more missed medical errors that could harm patients. The research appeared in JAMA Network Open.
As physicians use EHR systems, they often face pop-ups with alerts, reminders, and clinical care guidelines. But many doctors find these notifications to be more distracting than helpful, and could be designed better.
The researchers say their findings point to the need to improve EHR systems to make them easier to use and safer. Previous research showed that EHRs failed to reliably detect medical errors that could harm patients, including dangerous drug interactions. Experts had hoped that moving to EHRs would help reduce the number of people injured from medical errors – estimated to be as many as 400,000 annually.
Researchers studied EHR systems in 112 U.S. hospitals and compared results from an EHR experience survey taken by more than 5,600 clinicians with outcomes from an EHR safety evaluation tool.
According to the study, the user experience strongly correlated with EHR safety. When users rated a system poorly, common complaints were that the systems were difficult to operate, hard to learn, slow, or inefficient. When these complaints occurred, those EHR systems were less likely to flag drug interactions, patient drug allergies, duplicate orders, excessive dosing, or other medical errors.
The report notes that a lack of quality control may be to blame, because individual hospitals modify EHR operability to meet their specific needs, and some may sacrifice safety. There are currently no standards for EHR usability and safety, so even though health care providers may think the systems are safe, that may not be the case.
Researchers recommend that a collaborative effort among EHR vendors, hospitals, and physicians may help optimize EHR software for better usability and safety. [source]
ECU Health Medical Center Earns National Accreditation from Commission on Cancer

Greenville, N.C. – ECU Health Medical Center recently earned accreditation from the Commission on Cancer (CoC), a quality program of the American College of Surgeons (ACS). This accreditation means patients will receive comprehensive, personalized care provided by a team of specialists working closely together, access to information on clinical trials and new treatment options, ongoing monitoring of care and lifelong follow-up, mental health support, financial guidance, survivorship care and other long-term services.
“I am proud of our ECU Health team members and physicians who work hard to provide the most advanced cancer care to eastern North Carolina,” said Brian Floyd, president of ECU Health Medical Center and chief operating officer of ECU Health. “Cancer is a terrible disease that has touched most, if not all, of us in some way. As a regional academic medical center, ECU Health Medical Center strives towards excellence in all it does. This accreditation is a testament to the tireless efforts of our cancer care teams, who make a difference in the lives of so many in our region.”
The exterior of ECU Health Medical Center, near the Eddie and Jo Allison Smith Tower.
As a CoC-accredited cancer center, ECU Health Medical Center applies a multidisciplinary approach and treats cancer as a complex group of diseases that requires consultation among surgeons, medical and radiation oncologists, diagnostic radiologists, pathologists, and other health care professionals that specialize in caring for cancer patients. Cancer patients benefit from having access to clinical trials, screening and prevention events, palliative care, genetic counseling, rehabilitation, oncology nutrition, and survivorship services.
“Eastern North Carolina faces a disproportionately high rate of cancer, and as the largest health care provider in the East, ECU Health is committed to maintaining excellence in the delivery of comprehensive, compassionate, patient-centered, high-quality care for patients with all types of cancer,” said Dr. Darla Liles, Cancer Committee chair at ECU Health, professor and chief of the Division of Hematology and Oncology at the Brody School of Medicine at East Carolina University. “This accreditation demonstrates our holistic approach to cancer care that includes preventive measures, educational resources, clinical trials, support and survivorship services and treatment with the latest technologies and highest clinical standards.”
According to the American Cancer Society, more than 1.9 million new cancer cases and approximately 609,820 deaths from cancer are expected in 2023 in the United States. Of those, 67,690 new cases are expected in North Carolina. Residents in eastern North Carolina have access to ECU Health’s cancer care network that spans across nine hospitals – including the Eddie and Jo Allison Smith Tower at ECU Health Medical Center in Greenville that is home to both inpatient and outpatient cancer services – five radiation oncology sites, three joint ventures and numerous outpatient clinics.
“The Commission on Cancer brings together experts and advocates from across the country to develop standards for cancer care so that patients with cancer receive the highest quality care coordinated by a team of dedicated physicians and specialists,” said Timothy Wm. Mullett, MD, MBA, FACS, professor, general thoracic surgery medical director, Markey Cancer Center Affiliate and Research Networks University of Kentucky, and chair of the Commission on Cancer. [source]
TBT: Celebrating 20 years of Leadership in Medicine! Here is Dr. Scott Paviol's MEDTalk from 2018
The North Carolina Medical Society is celebrating 20 years of Leadership in Medicine!
Here is a Throwback Thursday to Dr. Scott Paviol's MEDTalk from 2018 on "Creating Space for Mindfulness"
Look for a new TBT every Thursday until the 2023 LEAD Conference and Golden Stethoscope Gala, October 13-14.
https://youtu.be/yYBIiFPrnrY
The 2023 LEAD conference registration is OPEN.
Click here for more information.
Please contact Erica Hall at NCMS to organize a class table at the LEAD Gala of your own.
Register Now! Fetal Alcohol Spectrum Disorder Awareness Webinar
September is Fetal Alcohol Spectrum Disorder Awareness Month

In honor of FASD Awareness Month, NCDHHS's Division of Mental Health, Developmental Disabilities, and Substance Use Services will host a webinar on September 27 from 12:30 to 2 p.m. to raise awareness for FASD and celebrate the strengths, talents and victories of the estimated one in 20 individuals who suffer from FASD.
This year marks the 50th anniversary of FASD Awareness Month. FASD is a term for a range of diagnosable conditions that can occur with prenatal alcohol exposure. Individuals with FASD exhibit higher rates of life challenges commonly encountered in substance use and mental health treatment populations, including higher risk of suicide, exposure to trauma, and increased interaction with criminal justice systems.