FDA Issues Revisions of Emergency Use Authorization for Paxlovid
The FDA recently announced a revision to the Paxlovid emergency use authorization (EUA).…
The Time is Now to Vaccinate Your Patients, FDA Greenlights Novavax Updated COVID Vaccine
Novavax COVID-19 Vaccine, Adjuvanted (2023-2024 Formula) is now authorized and ACIP recommended…
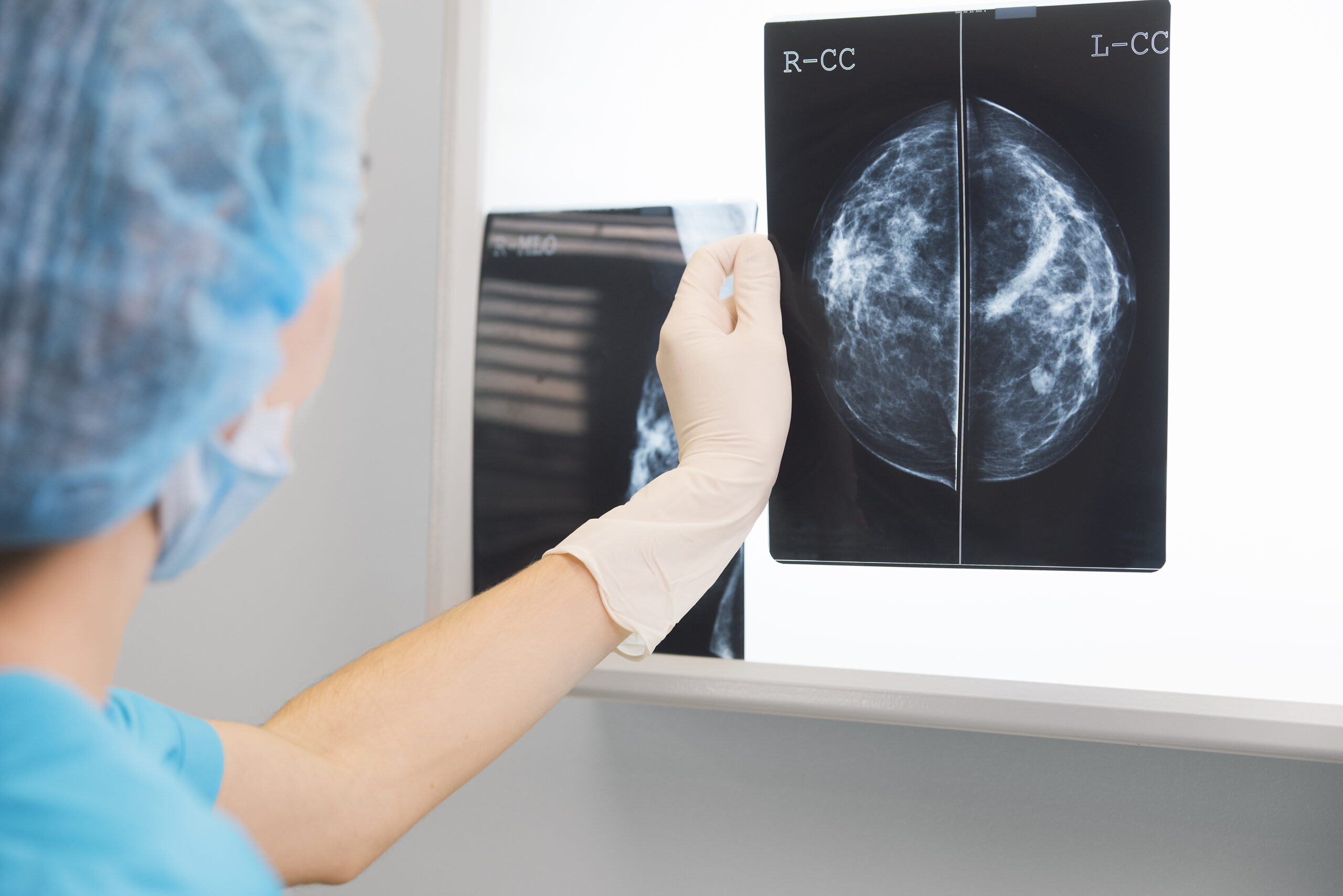
New Advancements Could Make Cure for Sickle Cell Disease a Reality
The FDA is considering the first CRISPR gene editing treatment that may cure sickle cell.
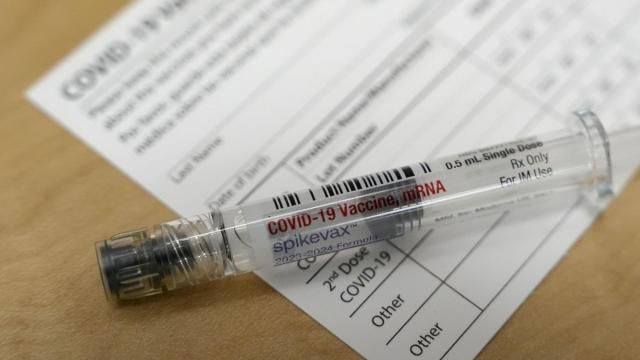
FDA: Children May Be Receiving Wrong Dose of New COVID-19 Vaccine
The FDA has issued an alert to health providers who provide Moderna's updated COVID-19 vaccine to…
FDA Warning: Stop Using These Over-the-counter Eye Drop Products
FDA warns consumers not to purchase or use certain eye drops from several major brands due to risk…
FDA Could Greenlight New COVID-19 Booster Any Day, Shots Will Not Be Covered by Federal Government
The shots could become available next week, after the Centers for Disease Control and Prevention…
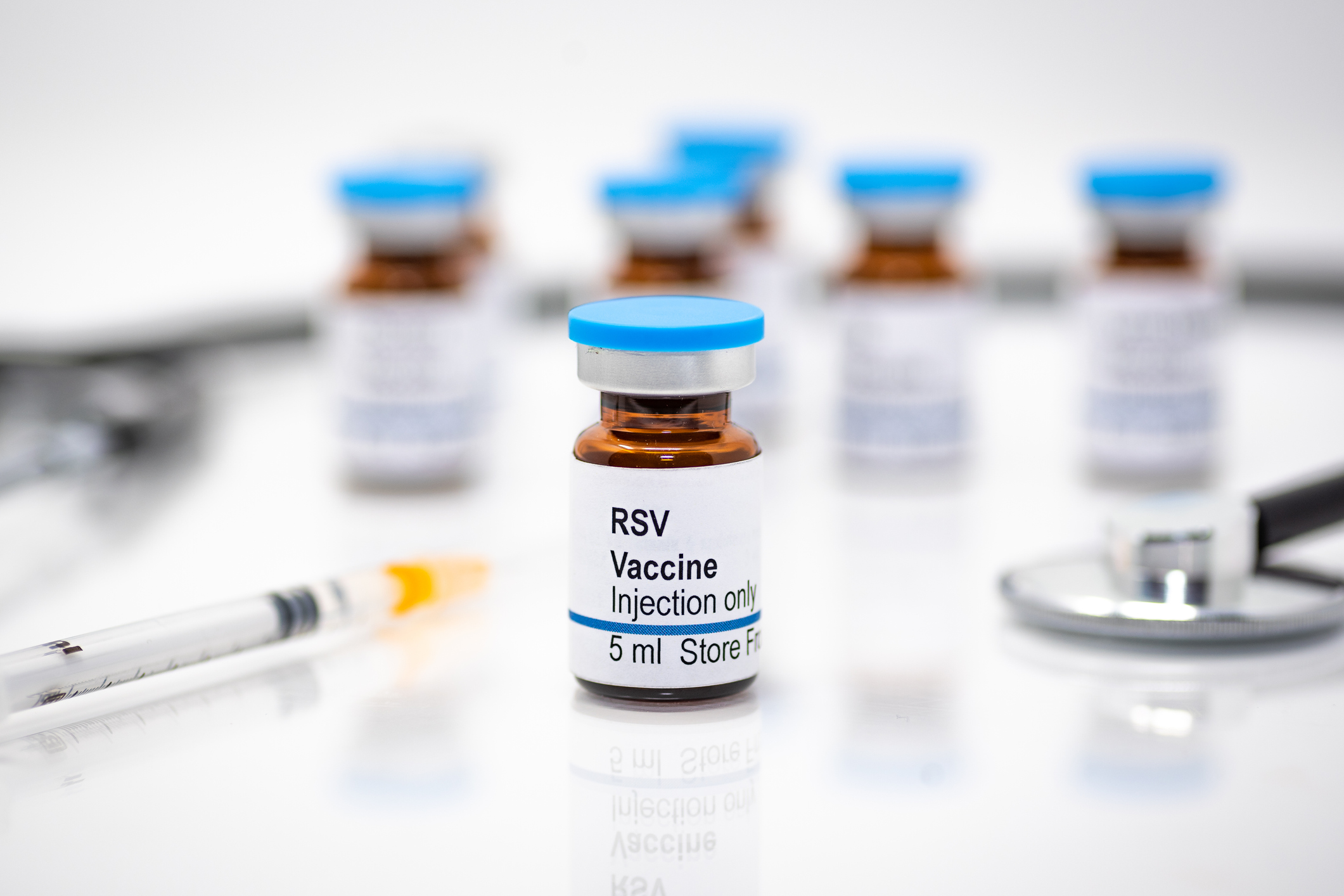
Questions About New RSV Option for Infants? Pediatric Experts Answer Questions in Live Panel Discussion
You're invited to join the RSV National Expert Program to learn about a new FDA-approved…
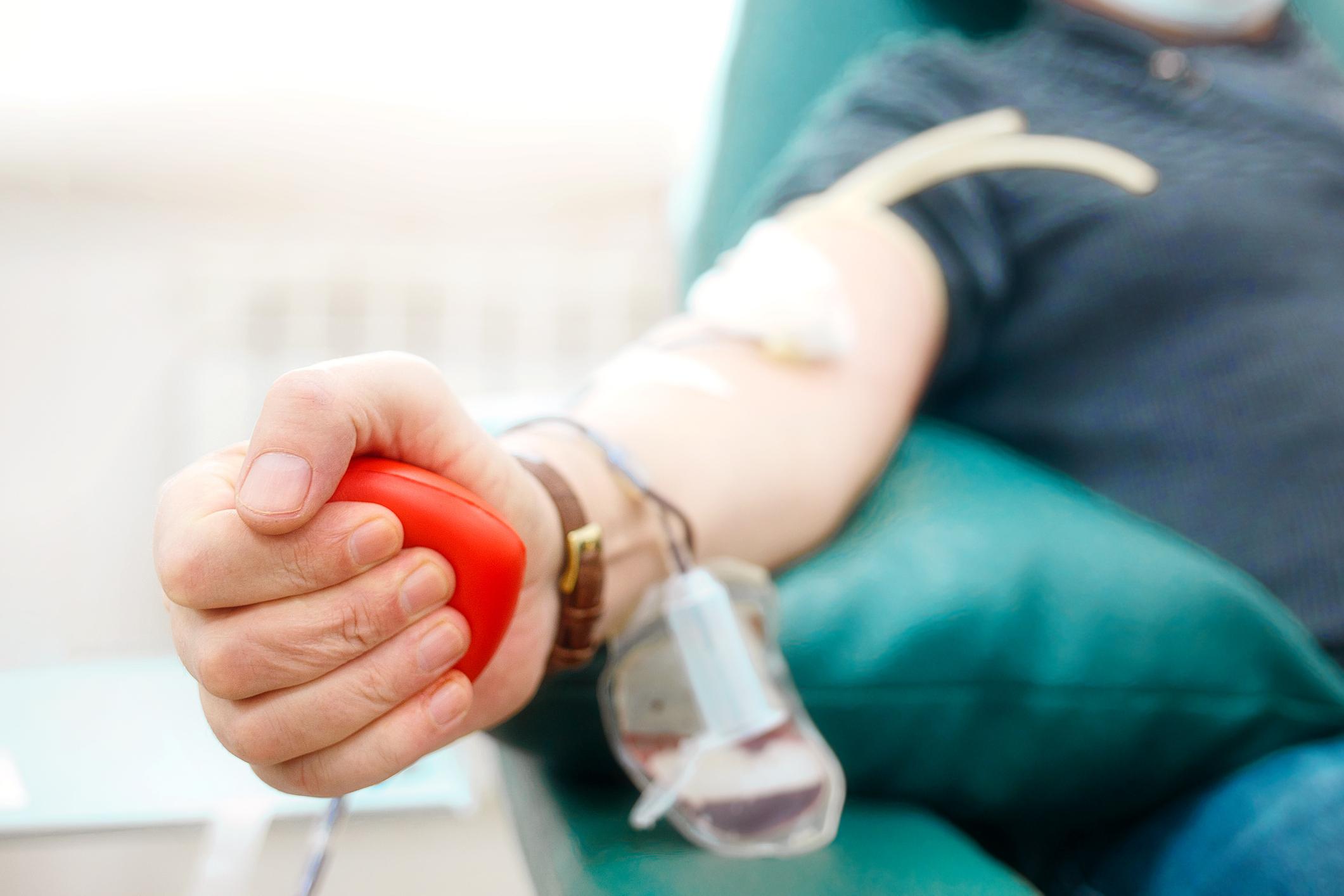
NCDHHS Secretary Kody Kinsley to Donate Blood, Celebrating Expanded Eligibility for Donations
Kinsley Joined Effort by Nine States and the District of Columbia to Push For Change
FDA: Allow Imports of Chemotherapy Drug From China to Ease U.S. Shortage
The agency will allow imports of the chemotherapy drug cisplatin, which is used to treat a wide…
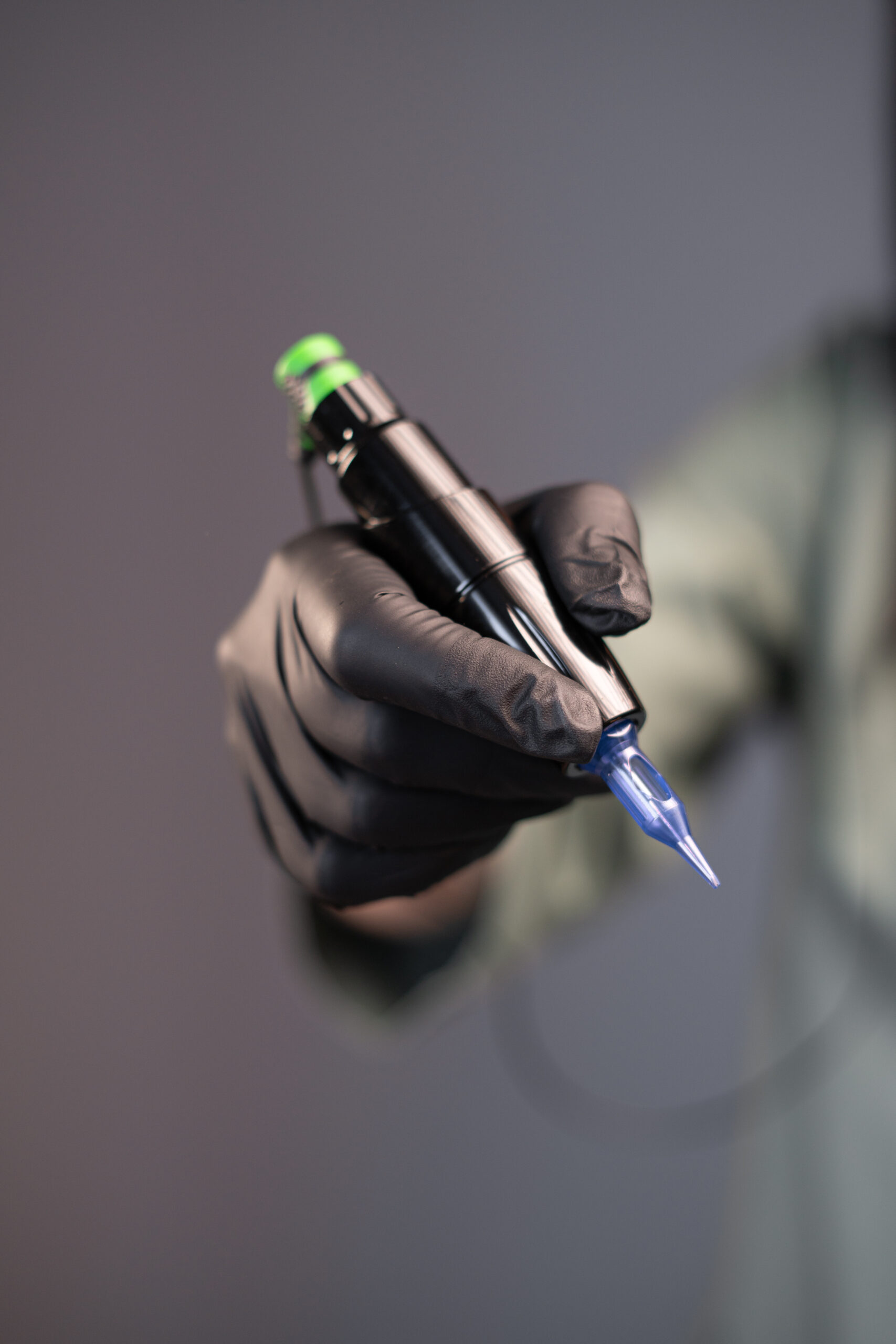
New Data Backs Safety of Semi-Permanent Ink for Use In Radiation Treatment
Semipermanent ink could soon be used in radiation treatment Researchers said early data backs…
Smartphones Could Be Next Step to Monitor Congestive Heart Failure in Patients
Smartphones can help doctors detect fluid accumulation via a patient’s voice
- 1
- 2